Cattle Diseases
Ostertagiasis in Cattle
Ostertagia ostertagi is a common roundworm that causes parasitic gastroenteritis in cattle.
Infection with Ostertagia can be classed as type I and type II.
Type I Ostertagia infection
This disease is most common in late summer and autumn and often causes profuse watery diarrhoea in calves at grass. The full parasitic life cycle can be seen on the picture on the right, but the infective L3 are ingested and they migrate to the abomasum, where they borrow into the gastric glands.
Here they undergo two moults (through L4 and L5 stage) and erupt out of the gland as an adult. This eruption from the gland is what causes pathogenesis (See image on the right). There is a rapid loss of weight, largely due to the loss in appetite. In chronic cases, bottle jaw (submandibular oedema) may result.
The direct cause of Type I ostertagiosis is the ingestion of large numbers of infective larvae over a relatively short period of time. The number of infective larvae on pasture is lowest in May and June, but rises to a peak in late August and September (See graph). This pattern arises from a sequence of events which starts with calves turned out in April or May onto pasture grazed by cattle (and especially calves) during the preceding year. These calves ingest some of the infective larvae which have overwintered, which may be a significant number (Dimander et al., 2003).
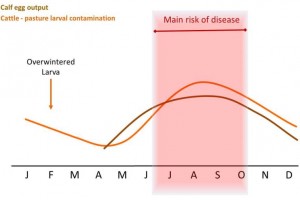
Some larvae will over-winter on pasture and be ingested by susceptible stock in spring. As a result larval pasture contamination will gradually increase through the summer, making late summer / autumn the main risk period for first season grazers.
In dairy herds typical cases of type I Ostertagia infection occur in spring-born calves turned out in midsummer onto pastures grazed and contaminated in the spring and early summer by autumn-born calves. In a survey, it was found that 65% of farmers used the same pasture each year for calves (Stafford and Coles, 1999), which is not recommended. The disease is not usually a problem in spring-calving beef herds, as the calves are too young to consume much grass in the early part of the season. As a result, the peak of infective larvae does not develop until September or October, when most calves are weaned and housed. However, autumn-born beef calves may suffer from type I Ostertagia infection in the absence of preventative measures. In areas where climatic conditions allow autumn-born calves to be turned out in March or early April, type I Ostertagia infection may occur 4 to 6 weeks after going to grass.
Type II Ostertagia infection and Epidemiology
Infective larvae ingested from September onwards undergo a change in their normal parasitic development, resulting in a period of delayed development at the early fourth larval stage (L4) while within the abomasal wall. This phenomenon is called hypobiosis thought to be brought about by drop in temperatures seen in late autumn / winter. In the late autumn, calves may harbour many thousands of such larvae in their abomasal wall. Type II ostertagiasis results when these inhibited larvae resume their development, usually from February to May, the emerging larvae causing the same lesions as those causing type I disease.
Both management and climate can influence the likelihood of Type II disease. If calves are moved from permanent grazing to aftermaths and back to the original grazing in the autumn, the pasture build-up of larvae on the original fields may be enough to cause many ingested L3 to arrest at early stage L4. Also, in very dry summers, L3 in faecal pats may not be released until there is sufficient rain to break down faecal pats, which may occur later in the grazing season, meaning that ingested L3 arrest as early stage L4.
Although adult cattle acquire immunity by the age of 18 months, it should be remembered that adult cattle may carry a small to moderate worm burden (Agneessens et al., 2000; Eysker et al., 2002; Murphy et al., 2006) which may contribute to the parasite load on a farm, as they have been shown to be patent infections (Borgsteede et al., 2000; Eysker et al., 2002).
Occasionally bulls grazing calf paddocks or cows suffering from immunosuppression due to other diseases (Orpin, 1994), such as fascioliasis, or close to calving (Armour et al., 1996) may suffer from type II ostertagiasis. In these cases, larvae which have been arrested at early stage L4 due to host immunity are able to develop into mature parasites. Adult cattle in conventional herds with a higher antibody response to ostertagia have been reported to have lower milk yields (Sanchez et al., 2002; Charlier et al., 2005) and non-treated dairy cows have been reported to spend less time grazing with a drop in yield compared to treated controls (Forbes et al., 2004).
- Some L3 infective larvae can survive the winter on pasture and in soil, the numbers are dependent on factors like temperature, moisture and snow cover. These larvae function to seed infection and contaminate pasture via multiplication in new season grazing animals. This may be a clinical infection (large numbers of larvae) but is usually subclinical.
- Most overwintering L3 on pasture die off by early–mid-summer. However, some L3 remain in the soil for longer periods. This is important to remember in grazing management.
- The speed of development from egg to infective L3 increases towards mid-summer as temperatures rise, therefore there can be a pasture peak in infective L3 in mid-summer (July).
- If sufficient L3 are ingested in the summer, clinical disease can be seen between July and October.
- Speed of development from egg to L3 decreases in the autumn.
- As autumn progresses, ingested L3 develop to early L4 stage and become inhibited. Calves may harbour many early L4 stage in the abomasum but remain asymptomatic.
- If there is synchronous development of early L4 stage into mature L5, clinical disease (Type II) may be seen in late winter or early spring.
- Asynchronous development of early L4 stage may not cause disease but may contribute to pasture contamination in the following spring.
- Acquired immunity is slow to develop, takes until at least the end of the first grazing season and is dependent on the level of exposure in the first grazing season (Eysker et al., 2000).
- In spring calving suckler beef herds, grazing immune cows with calves at foot act to remove a large number of L3, therefore clinical disease is rare. However, in autumn calving beef herds, calves turned out for their first grazing season may ingest sufficient L3 to cause clinical disease especially if they are weaned.
Control and Prevention of Ostertagiasis
Control and prevention of parasitic gastrointestinal nematodes is covered in depth on the Parasitic Gastroenteritis in Ruminants page.
Matrix for risk assessment from Andy Forbes from the British Cattle Veterinary Association
| Risk factor | High | Medium | Low |
| Age (grazing seasons, GS) | < 1 year (First GS) | 1-2 years (Second GS) | > 2 years (adult)* |
| Age at turnout (Weaned calves, first GS) | < 6 months | 6-8 months | > 8 months |
| Weight gain (> 2 years old)
2 months after turnout |
< 0.7 kg/day | 0.7-0.8 kg/day | >0.8 kg/day |
| Faecal worm egg count (FGS)
2 months after turnout (epg) |
>200 | 50-200 | <50 |
| Herbage mass kg DM/ha
Sward height cm |
<1000
<4 |
1000-2000
4-8 |
>2000
>8 |
| Field type | Permanent pasture | Silage/hay aftermath | Newly sown fields |
| Grazing history | Grazed by cattle <1 year old within the last year | Grazed by cattle 1-2 years old within the last year | Grazed by adult cows, sheep** or other species within last year |
| Bulk milk tank O. ostertagi antibodies (ODR) (dairy herd) | >0.8 | 0.5-0.8 | <0.5 |
Treating ostertagiasis
The clinical signs of PGE in cattle include of ill-thrift and diarrhoea and may confirm diagnosis by taking a faecal sample to conduct a faecal egg count (FEC), by taking blood samples for blood pepsinogen measurements or by post mortem examination. Faecal egg counts are not always reliable in making a clinical diagnosis as the correlation between worm burden and egg output in cattle is low (Eysker and Ploger, 2000) and blood pepsinogens may be more reliable. However, FECs above 300 eggs per gram would usually be of concern.
Good Practice Based on Current Knowledge
- Parasite control should form part of the Herd Health Plan
- Avoid grazing first season grazing cattle on the same pasture each year
- Lower stocking density
- Regularly (monthly) monitor faecal egg counts through the grazing season in first and second grazing season animals. This is a useful way of monitoring the parasite burden in groups of animals, but may be of more limited value in individual clinical cases
- Consider ploughing and re-seeding pastures if possible, if the farm can include an arable ley to reduce the worm burden on grazed pastures
- Quarantine any new animals coming onto the farm to reduce the risk of introduction of anthelmintic resistance. See COWS guidelines for importing new stock


 American English
American English



Comments are closed.