Cattle Diseases
Salmonellosis in Cattle
Salmonellosis is a bacterial, zoonotic disease of humans and animals.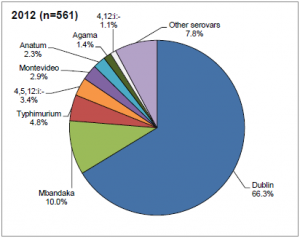 The most common serovar infecting cattle in the UK is Salmonella dublin (66.3%) followed by S. Mbandaka (10%) and S. typhimurium (4.8%) (DEFRA, 2012). The condition has different manifestations in infected animals. An acute generalised infection is seldom seen, but causes a severe condition that can be fatal without effective treatment in vulnerable individuals. An acute intestinal infection causes diarrhoea, particularly in young animals. Calves with Salmonellosis may become septicaemic and die, or may suffer from necrosis of extremities as a sequel, especially the feet, tail and ear tips. Other manifestations can be joint ill or pneumonia. A chronic intestinal infection can be asymptomatic clinically, and often leads to the presence of carrier animals that are not identified as Salmonella infected. Several serovars have been associated with abortions, with Salmonellosis being the third most commonly diagnosed infectious cause of abortion in the UK (approximately 10% of diagnosed abortions). Within a herd the spread is often rapid among the calves. Cattle movement, especially calf trade, also spreads the infection between farms.
The most common serovar infecting cattle in the UK is Salmonella dublin (66.3%) followed by S. Mbandaka (10%) and S. typhimurium (4.8%) (DEFRA, 2012). The condition has different manifestations in infected animals. An acute generalised infection is seldom seen, but causes a severe condition that can be fatal without effective treatment in vulnerable individuals. An acute intestinal infection causes diarrhoea, particularly in young animals. Calves with Salmonellosis may become septicaemic and die, or may suffer from necrosis of extremities as a sequel, especially the feet, tail and ear tips. Other manifestations can be joint ill or pneumonia. A chronic intestinal infection can be asymptomatic clinically, and often leads to the presence of carrier animals that are not identified as Salmonella infected. Several serovars have been associated with abortions, with Salmonellosis being the third most commonly diagnosed infectious cause of abortion in the UK (approximately 10% of diagnosed abortions). Within a herd the spread is often rapid among the calves. Cattle movement, especially calf trade, also spreads the infection between farms.
Patterns of Salmonella Infection
The majority of Salmonella isolations in the UK are associated with clinical disease as there is no routine surveillance for Salmonella. However, it has been found in other countries that many dairy farms may harbour Salmonella, either in the environment (up to 93% of herds tested) or as sub-clinical cases where shedding is from the cows themselves (10% of cows), thus, the farm environment should be viewed as an important potential reservoir of infection (Blau et al., 2005; Callaway et al., 2005; Fossler et al., 2005a; Peek et al., 2004). A study of organic and conventional dairy herds in the US found no difference in Salmonella prevalence between farm types (Fossler et al., 2005a).

Heifers infected between 1 year and their first calving have been reported to be at higher risk of becoming carriers
Salmonella dublin outbreaks often occur when “carrier” animals start excreting the agent after calving or in association with some other type of stress (liver fluke infection, ketosis). An infected dam will usually infect her calf immediately after birth. A low level of Salmonella isolations have been found in the lairage and in healthy animals at slaughter, with the prevalence of faecal excretion ranging between 0.2-2% of samples (Davies et al., 2004; McEvoy et al., 2003; Small et al., 2003). Additionally, salmonellae (mainly S. dublin) have been found on hides of animals in 7-17% of carcasses, (McEvoy et al., 2003; Small et al., 2002), which may be an important source of contamination of the food chain. Dairy cattle and feedlot cattle have been shown to more likely to shed Salmonella in faeces than pasture beef cattle (Vanselow et al., 2007).
Heifers infected between 1 year and their first calving and cows infected at calving have been reported to be at higher risk of becoming carriers (Nielsen et al., 2004) which suggests that good heifer and periparturient cow management can help reduce disease risk.
The shedding prevalence varies widely between different groups of cattle on any one farm, thus, careful testing must be undertaken (including environmental sampling) to ensure that the herd’s status is accurately determined and false negative results avoided (Warnick et al., 2003).
Salmonella Risk Factors
Salmonella can be introduced to a herd via a number of routes, particularly by the introduction of infected (usually sub-clinical) animals, or from contaminated feed (Davis, 2003). Once a herd is infected, contaminated slurry and animal waste can maintain the infection and lead to spread to neighbouring farms, particularly when spray spreaders are used.
Davison et al. (2006) identified several risk factors for salmonella on UK dairy herds: increased risks were observed with increased herd size, employing part time workers, lack of a clean visitor parking area and feeding milk powder instead of whole milk.
Livestock Related
Outbreaks caused by unidentified “carrier” animals starting to excrete the organism are recognised as a common cause of apparent “new” Salmonella infections in closed herds. Calf rearers with herds of multiple origin have often been identified as particularly at risk from Salmonella infections, with the calf trade in the UK considered to be endemically infected, (Wray et al., 1987b, 1991). Outbreaks have, however, been diagnosed in beef suckler herds as well (Davies and Renton, 1992). Outbreaks in dairy herds are often seasonally related to calving season, occurring in calves between two and six weeks of age. Shedding of Salmonellae in dairy cows has been reported to increase in cows at calving time and in larger herds (Fossler et al., 2005b). Direct contact between cattle from different farms has been shown to increase the risk of disease entry (Shaik et al., 2002).
Environment or Management Related
Wild birds (in particular starlings) have been identified as a potential risk factor for the introduction of Salmonella infection to farms and feed mills (Refsum et al., 2002, Carlson et al., 2015). Salmonella infections in neighbouring farms, storing feeds in open areas, access of cattle to surface water, disposal of wastes as slurry rather than manure, buying-in manure, a non-seasonal calving pattern and pooling of colostrum have also been reported as risk factors for new Salmonella infections (Fossler et al., 2005c; Veling et al., 2002; Wedderkopp et al., 2001). Additionally, livestock waste from animals consuming principally grass has been shown to be less likely to harbour Salmonella contamination compared to other diets, which may be of consequence in pasture based systems (Hutchison et al., 2005a).
Maintenance of Infection
In a longitudinal survey of cattle farms with salmonellosis, it was found that domestic pets, feral cats and wildlife could transmit the infection within the farm. Mice and birds were identified as long term carriers of the infection and feed and grain store security and hygiene were considered important in the process of eradicating salmonellosis from a farm (Davies, 1997).
Important Zoonotic Aspects of Salmonellosis in Cattle
Cattle have been identified as the main animal source of S. typhimurium infections in humans in the UK (Calvert et al., 1998). Potentially, spread from cattle to humans can be via direct contact, contamination of the environment (i.e., into water courses) and contamination of meat and milk products.
Salmonella isolation from food producing animals has to be reported by the laboratory isolating the bacteria to the Senior Veterinary Investigation Officer in the local VLA centre in England and Wales, or to the Divisional Veterinary Manager in Scotland under the Zoonoses Order of 1989.
Control and Prevention of Salmonellosis
To maintain a herd free of Salmonella infection, a closed herd policy and high standards of hygiene, including prevention of contamination of feed by birds and wild animals, are necessary. This should be discussed with the farm’s veterinary surgeon and should form part of the biosecurity component of the Herd Health Plan. If purchased animals are introduced into the herd, cattle markets and dealers’ yards should be avoided (Wray et al., 1990). If animals need to be transported between farms, it is advisable to use the farm’s own vehicle and disinfect the wheels and the vehicle after use (Wray et al., 1991).
The following risk factors within the farmer’s influence relevant to salmonellosis control on a farm were identified by (Evans, 1996):
- Purchasing replacement stock from direct sources rather than from dealers
- Quarantine of purchased cattle for 4 weeks before inclusion in the main herd
- Housing sick animals in isolated areas
-

Storing slurry for at least a month before spreading reduces the risk of infection as does delaying grazing for 6 weeks after spreading
Preventing wild bird access to feed stores
Whilst slurry and sludge spreading on pasture has not been associated epidemiogically with salmonellosis in cattle or humans, Salmonellae do survive long periods of time both in slurry and in herbage after spreading. It is recommended that the risk of infection can be significantly reduced by storing the slurry at least for a month before spreading it and by delaying grazing for six weeks after spreading slurry on a pasture (Jones, 1976). Good composting technique, allowing a sufficient rise in temperature in the compost to develop, has been found to rapidly decrease Salmonellae and should form part of the preventive approach (Hutchison et al., 2004, 2005a, 2005b, 2005c; Lung et al., 2001).
The eradication and control of salmonellosis on a persistently infected farm is difficult. Faecal or serological sampling of all animals with group and individual samples and identification and slaughter of carrier animals has been used successfully in voluntary salmonellosis control in Finland (Nieminen 1996; Jensen et al. 1994). Combined vaccination and faecal identification and culling of infected animals has also been used with varying success (Davies and Renton, 1992; Hunter and Peek, 1977). All authors stress the importance of sanitary and husbandry measures alongside with the above-mentioned control strategies. The most important of the sanitary measures mentioned appears to be the prevention of feed contamination either during feeding or in the feed stores.
It is important to remember the potential risk of spread of infection via contaminated milk on dairy farms, therefore, milk from affected animals should not be fed to calves and raw bulk tank milk should not be consumed by farm personnel due to the zoonotic risk. Where salmonella in calves is a continuous problem, on-farm pasteurisation of milk will reduce the spread.
Treatment of Salmonellosis
The treatment of Salmonella infection in cattle with antibiotics in acute cases is common and may reduce mortalities if initiated early and combined with support therapy. Animals which have received antimicrobial therapy have been reported to be less likely to shed Salmonellae, so this may help reduce cross infection in the cohort (Fecteau et al., 2003; Fossler et al., 2005d). In contradiction to this, Warnick et al., (2003) found a higher risk of shedding in heifers and cows but not calves which have recently received antimicrobial treatment. The use of antibiotics metaphylactically in the face of an outbreak is not recommended, due to the high risk of antimicrobial resistance development. Prophylactic antibiotic administration in feed does not appear to have any effect on excretion of Salmonellae in calves either (Wray et al., 1987b). In an outbreak, appropriate support therapy for severely affected animals and vaccination can be helpful. Rehydration therapy and Salmonella antiserum (where available) have been found helpful in preventing high mortalities.
Isolation of affected animals, careful avoidance of contamination of any feed with faeces, disinfection of all utensils, tools and protective clothing used, and possible identification of the contamination source are important.
Welfare and Salmonellosis
A Salmonellosis outbreak in a dairy or beef herd can lead to severe disease, particularly among pregnant females, who may abort. It is important to avoid bringing in the disease, as treatment and control options are difficult and limited. Animals with acute disease symptoms must receive veterinary attention.
Good Practice Based on Current Knowledge
In the case of a Salmonella-free herd:
- Stick to a closed herd policy
- Diagnose all cases of diarrhoea or abortion by sampling and submitting to laboratory analysis
- If animals need to be bought in, avoid dealer’s yards and cattle markets: buy directly from the farm of origin and find out the disease status beforehand
- If the disease status of the farm of origin is not clear, quarantine bought-in animals for 4 weeks before including in the main herd
- Avoid bringing in contamination from other farms (i.e. on borrowed machinery)
- Make sure that the feed storage is secure and there is no access for wildlife or domestic pets.
- Communicate with your neighbours!
In the case of a herd with an ongoing Salmonella infection:
- At the start of an outbreak, consider vaccinating animals, as this has been shown to offer some protection.
- Employ strict hygiene practices and isolate sick animals
- Consider a whole herd test programme with culling of persistently positive animals
- Do not sell animals from the farm – other than direct to slaughter – until the farm is cleared, unless you tell the buyer about the ongoing salmonellosis on the farm
- Communicate with your neighbours!


 American English
American English


Comments are closed.