Cattle Diseases
Ketosis
Also known as: Acetonaemia, Fat Cow Syndrome, Hypoglycaemia and Pregnancy Toxaemia.
Primary ketosis, or acetonaemia, is a metabolic disorder and is largely a disease that is influenced by management of dairy cows in early lactation. Ketosis is an important clinical and subclinical disease, as there are several metabolic disorders and diseases that commonly occur in the calving and the early lactation period that are linked to ketosis (including milk fever, retained foetal membranes and displaced abomasum). Hypoglycaemia is the major factor involved in the onset and development of clinical ketosis. There is a gradual loss of body condition over several days or even weeks. There is also a moderate to marked decline in milk yield (up to 5 litres per day) over five to six days before the onset of obvious clinical signs (Edwards and Tozer, 2004). This can persist for up to two weeks after diagnosis (Rajala-Schultz et al., 1999). The disease is most commonly seen in high-yielding dairy cows in early lactation. Secondary ketosis due to lack of appetite as a result of another disease can be seen at any stage of lactation. Beef cows may also suffer from ketosis during pregnancy, although this is less commonly recognised.
Primary ketosis in dairy cows
Pathogenesis of Ketosis
To satisfy the requirements of milk production, the cow can draw on two sources of nutrients – feed intake and body reserves. During early lactation, the energy intake is insufficient to meet the energy output in milk and the animal is in a negative energy balance. In conventional farming, this is considered to be a normal metabolic situation in high-yielding dairy cows. Cows in early lactation are, therefore, in a vulnerable situation, and any stress that causes a reduction in feed intake may lead to the onset of clinical ketosis.
If the feed intake of the cow is not sufficient to meet the demand for energy, there is insufficient ruminal production of propionic acid (also known as propanoic acid), the main precursor of glucose in ruminants, which results in hypoglycaemia. Hypoglycaemia leads to a mobilisation of free fatty acids and glycerol from the fat stores which are oxidised. However, the liver cannot deal with the Acetyl-CoA, which results from the oxidation of these fatty acids, because of a lack of energy. The excess acetyl-CoA is converted into the ketone bodies acetoacetate and β -hydroxybutyrate and, to a small extent, acetone (See image).
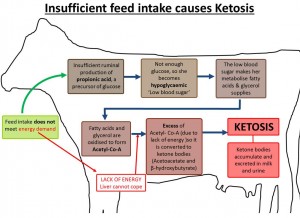
Ketosis diagram showing how ketosis is caused when the feed intake does not meet energy demands, causing hypoglycaemia, and the knock-on effects resulting in ketosis.
Tissues other than the liver can use ketone bodies, but if their production exceeds the rate at which they are used by muscle and other tissues, they accumulate, and ketosis is the result. Ketone bodies are excreted in milk and urine (Andrews, 1998; Holtenius and Holtenius, 1996).
The reduction of propionic acid production is usually the result of underfeeding or a reduced feed intake caused by reduced appetite or lack of feed (quality, quantity and access). A period of inappetance is normal around calving, but may be worsened in the early post-partum period by a deterioration of forage quality, sudden changes in diet or excessive fatness at calving (Andrews et al., 1991; Higgins and Anderson, 1983). Other risk factors are:
- The season, ketosis is more likely in winter
- Increasing parity
- Cows with milk fever have a greater decrease in feed intake after calving which exacerbates the negative energy balance, increasing the risk of ketosis (Goff and Horst, 2003).
- Ketosis in the previous lactation
- Increased 305-day milk yield in the previous lactation
- The average milk protein percentage in the previous lactation (Heuer et al., 2001; Rasmussen et al., 1999; Wood et al., 2004).

When silage contains too much butyric acid, it can cause ketosis as butyrate is a precursor of Acetyl-Co-A, and it is a build-up of this molecule that leads to the production of ketone bodies and ketosis. Forage quality should be checked several times every year.
Butyrate is a precursor of acetyl-CoA and is therefore ketogenic. Butyric acid content of silage is of some importance in causing the disease, because wet conditions predisposes butyric fermentation of the silage. Silage containing high levels of butyric acid is also less palatable to cattle (Andrews et al., 1991).
Clinical Signs of Ketosis
Cows will have raised blood ketone levels and may excrete ketones in urine and milk. It is important to recognise that many cases of ketosis are subclinical, with the cow’s performance and health compromised, but without visible clinical signs. The clinical signs of ketosis include a refusal to eat grain and concentrate feeds and a sudden drop in milk output. There is a sweet smell of acetone in the breath and milk. Some cows may exhibit nervous signs, which include excessive salivation, abnormal chewing movements, licking of walls, gates or metal bars, incoordination with apparent blindness and a degree of aggression. The nervous signs often only last for a few hours. The affected cows have fatty infiltration and degeneration of the liver (Moore and Ishler, 1997). Cows with ketosis are at greater risk of developing retained foetal membranes, displacement of the abomasum (Geishauser et al., 2000; Melendez and Risco, 2005) and are more likely to have prolonged calving to conception intervals and lower fertility (Fourichon et al., 2000; Gillund et al., 2001; Opsomer et al., 2000). Due to an impaired immune system they are also more susceptible to certain types of mastitis (Janosi et al., 2003).
Secondary Ketosis
Secondary ketosis is common and is the result of diseases causing a reduction in appetite in early lactation, such as displaced abomasum, mastitis and metritis. In areas of cobalt deficiency , ketosis may be diagnosed in grazing cattle. Cobalt is required for rumen microbes to synthesize vitamin B12 and is also essential for adequate utilization of propionic acid (Eddy, 2004). Ketosis has also been reported as the major sign in a dairy herd with liver fluke or fasciolosis (Mason, 2004).
Ketosis in Beef Cows

In suckler herds over-fat cows are at more risk of developing ketosis in late pregnancy, usually around 7-9 months. Energy intake must meet energy demands to prevent ketosis and cows must not be too fat around calving.
Whereas ketosis in early lactation mainly affects high-yielding dairy cows, ketosis or pregnancy toxaemia in late pregnancy mainly affects beef cows. Cows of all ages can be affected, but over fat animals and those carrying twins are most at risk. Beef cows often get over fat on good summer pastures. If the same cows do not have access to high quality forage during the winter months, when they are in late pregnancy, they will succumb to ketosis due to a low energy intake. Affected cows are usually 7-9 months pregnant, and the initial clinical signs are the same as for ketosis in early lactation. However, many cows become recumbent fairly quickly and most die three to fourteen days later. Cows affected close to parturition often die during parturition (Caple et al., 1977; Morris et al., 1992; Spence, 1978).
Control and Prevention of Ketosis
One of the guiding principles of sustainable livestock production is to feed high levels of roughage in the diet so as to promote good rumen digestion. For dairy cattle fed at least 60% fresh or conserved roughage, this should be of high quality during early lactation to meet the energy and protein requirements. This is especially important in the winter diet based on home-gown, conserved forages and it may be difficult to supply sufficient energy if cows are high yielding (Bystrom et al., 2002; Trachsel et al., 2000).
Transition cow management (the late dry period up to the first 1-2 weeks of lactation) is critical in prevention of a range of metabolic diseases including ketosis, and as such should be highlighted in the herd health plan. The aims of the transition period are to allow cows to develop a strong immune system, maintain normal concentrations of calcium in the blood, minimise the negative energy balance, prevent calving related diseases and develop a rumen adapted to the post-partum diet (Melendez and Risco, 2005). The following points should be considered:
- Cows should not be too fat at calving, as this depresses their feed intakes. A condition score of 2.5-3.0 on a 1-5 scale is optimal, and anything higher is considered too fat and at greater risk of ketosis (Gillund et al., 2001; Melendez et al., 2004)
- Concentrates fed during lactation should be introduced in small amounts, approximately two weeks before calving, to allow adjustments of the rumen microflora. Dietary changes during early lactation should be made gradually
- Efforts should be made to ease the transition from gestation to lactation by offering highly palatable forage at calving and providing suitable accommodation and assistance where necessary (Grummer et al., 2004; Moorby et al., 2002)
- Roughage high in butyric acid should be avoided in early lactation. Forage quality should be checked several times every year. In cobalt deficient areas, measures should be taken to ensure adequate cobalt intake (Eddy, 2004)
- A sufficient supply of high quality forage is essential for beef cows in late pregnancy
- Metabolic profiles using blood samples taken from groups of dry cows and cows in early lactation can help vets monitor the herd health and detect subclinical disease. Dietary changes can then be made if necessary to reduce disease. Milk tests are also being developed to test for ketosis (Jorritsma et al., 1998)
- The heritability for ketosis is relatively high, and this trait should be used to advantage (Van Dorp et al., 1998; Zwald et al., 2004) Additionally, there may be some variation in breed predisposition to ketosis (Schnier et al., 2004) although management has a much greater effect on farm than breeding
- In the UK bull proofs a fertility index has been developed which can indirectly be used to reduce ketosis, as some of the factors contributing to it (milk yield and body condition score) are linked to the ketosis
- Housing also seems to have an effect on the level of ketosis in the herd. Research from Norway indicates that a significantly higher proportion of herds with tie stalls experienced ketosis compared to those with free stall herds (Valde et al., 1997). The reason for this is not known but may lie in the fact that muscles can utilize ketone bodies
Treating Ketosis
Diagnosis is confirmed by the clinical signs of milk drop, reduced appetite and elevated ketones in the blood, urine or milk.
There are three main aims of treatment:
- To restore blood glucose levels as quickly as possible
- To replenish oxaloacetate, the essential intermediate in the tricarboxylic acid cycle (TCA), so that fatty acids are completely oxidised and the production of ketone bodies reduced
- To increase the availability of dietary glycogenic precursors, notably propionic acid (Eddy, 2004)
Intravenous glucose therapy with 500 ml 40% glucose will give a short term rise in blood glucose levels that will only last 2 hours. It should be accompanied by oral administration of a glucose precursor, preferably propylene glycol. Administer propylene glycol twice daily for three to four days. Cobalt salts may be added to propylene glycol.
In conventional farming, glucocorticoid drugs are commonly administered as therapy, used either alone, or in combination with glucose therapy, or followed by oral administration of glucose precursors. Glucocorticoids help to stimulate gluconeogenesis.
It is important that the cow’s appetite is returned to normal as soon as possible after treatment, so access to high quality fodder is important. Provision of highly palatable feed, such as molassed feed can help encourage the cow to eat.
Most cows with ketosis in late pregnancy (pregnancy toxaemia) are so severely affected that medical treatments invariably fail. Immediate removal of the calf by induction or caesarean section may save the cow. This should be followed by the treatments mentioned above (Eddy, 2004).
Good Practice Based on Current Knowledge
Prevention of ketosis is important, as cows with clinical and sub-clinical disease have a reduced milk yield and are predisposed to a number of other conditions due to immunosuppression. The key to prevention of ketosis is good transition cow management.
- Check forage quality several times every year
- Beef cows should receive adequate levels of high quality forage to prevent ketosis (pregnancy toxaemia) in late pregnancy
- Feed high quality roughage during early lactation
- Use cows suitable to the farm system, i.e., do not breed for high yields in isolation
- Ensure cows are not too fat at calving (condition score 2.5-3.0)
- Introduce concentrates in small amounts approximately two weeks before calving
- A suggested dry period ration would contain 50% of the lactating cow ration and 50% straw, to give an ME of 9MJ/kg DM and 14% CP
- Avoid major dietary changes during early lactation
- Avoid roughage high in butyric acid in early lactation
- Use regular body condition scoring throughout the lactation. Look out for sudden changes in trends
- Ensure that all cows have good access to feed, i.e., enough feeding space for the lower ranking cows. Pay particular attention to first lactation cows
- Ensure adequate cobalt intakes
- Take metabolic profiles in groups of dry cows and cows in early lactation to check whether the diet is adequate and maintain assurance that transition cow management is optimal


 American English
American English


Comments are closed.