Cattle Diseases
Mastitis in Cattle
Also known as: Udder Inflammation
Although there is limited information on mastitis in beef herds, summer mastitis and environmental pathogens can be a problem in some cases, particularly if calving indoors. The general information and advice given in the rest of this chapter can generally be applied to beef herds with some modification to the different systems.
Causes and Risk Factors
Host Determinants for Mastitis
Environmental and Management Determinants
Control and Prevention
Treatment Options
Good Practice
Mastitis and Somatic Cell Counts (SCC)
Somatic cell counts (SCC) consist mainly of immune cells that enter the milk compartment of the udder. There are always small quantities of immune cells in the cow’s milk, and their function is to protect the udder against infection by bacteria. The older the animal gets, the more somatic cells it tends to have in its milk. Similarly, the SCC levels are higher immediately after calving and towards the end of each lactation period. SCC have long been used as a way of measuring milk quality. Most dairy companies base their milk pricing policy, among other things, on SCC values of the milk. Individual cow SCC are widely used to identify cows likely to have an intramammary infection, and bulk milk somatic cell count (BMSCC) is used to estimate herd mastitis prevalence (Madouasse et al., 2010). Mastitis impacts on milk quality in terms of increased SCC and can reduce protein and fat levels.
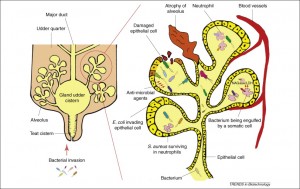
Udder diagram illustrating how mastitis develops in an infected udder. Environmental and contagious microorganisms invade the udder though the teat cistern. They then multiply within the alveolus where they are attacked by neutrophils (white blood cells) while damaging the milk-producing epithelial cells of the bovine udder. The image is taken from Viguier et al, 2009.
When bacteria do enter the udder, the number of immune cells increases rapidly, as the immune system attempts to overcome the infection. Once the infection has been cleared, the SCC levels gradually drop to normal. However, this can sometimes take weeks. In cases of chronic infection, where the bacteria persist in the udder, the SCC levels can remain high throughout the lactation, or may fluctuate if an infection recrudesces.
High SCC levels in the milk cause deterioration of the milk quality. It has been shown that levels above 500 000 cells/ml decrease cheese yield and affect yoghurt making (Politis and Ng-Kwai-Hang, 1988). Consistently high SCC levels in a herd are usually a sign of high levels of subclinical mastitis. Most cases of subclinical mastitis are caused by gram-positive bacteria such as Streptococcus uberis and Staphylococcus aureus. Streptococcus agalactiae has been eradicated from most dairies, but can cause severe problems if re-introduced.
Mastitis in the UK
A recent estimated incidence of clinical mastitis in England and Wales is 47 cases per 100 cows per year (Bradley et al., 2007). Mastitis rates tend to increase in the winter (Ellis, 2005; Whitaker, 2002) although conversely, SCC tend to increase in the summer (Ellis, 2005).
Whilst mastitis control programmes have had a major impact on the improvement of udder health in the British national dairy herd, it has been suggested that introduction of milk quota systems within the EU, quality payments based on bulk milk SCC and legislation setting maximum SCC levels for saleable milk within the EU have been the major encouraging and motivating factors in bringing about a reduction in SCC over the past 30 years. It has also been pointed out that the incidence of clinical mastitis has not declined at the same rate as the SCC in the past 15-20 years, but has rather remained at an endemic level in the national herd (Booth, 1997). However, clinical mastitis is not centrally recorded, and on-farm records vary widely in quality, therefore, all estimates of clinical incidence need to be treated with care.
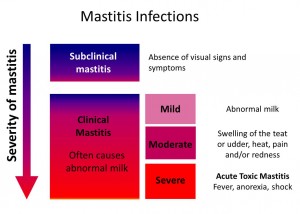
The first step in tackling a case of clinical mastitis is to determine the severity level. Once this is determined, the farmer can decide on the course of action albeit obtain an SCC (if don’t routinely), carry out a bacterial examination, close monitoring, or treatment.
Types of Mastitis and Clinical Signs
There are four main clinical presentations of mastitis recognised:
- Severe and acute toxic mastitis
- Mild/moderate clinical mastitis
- Sub-clinical mastitis
- Summer mastitis
Severe and Acute Toxic Mastitis
This type of mastitis is often seen in cows in the period soon after calving and may be more frequent in cows with lower SCC. It is primarily caused by Gram-negative, endotoxin producing bacteria, such as E. coli, although in some cases S. aureus can be the pathogen. This type of mastitis is very severe and may kill cows unless they are treated promptly. The signs are primarily due to the endotoxin produced by the bacteria, causing the cow to develop endotoxic (also known as toxaemic) shock. The cow will present as acutely sick, dull, and may be recumbent. She may have a high temperature or conversely may have a sub-normal temperature if in shock. The udder may show a variety of changes including swelling, heat, pain and the milk may look watery and discoloured though often not with ‘clots’. The cow must be immediately treated by a vet.
Mild/Moderate Clinical Mastitis
This type of mastitis is what is commonly recognised as a clinical case. At milking time the cow is often noticed to have abnormal milk (mild case), and in moderate cases a swollen udder, affecting one or more quarters. The cow is not systemically ill and appears bright and eating normally. She may show some signs of discomfort when walking due to a distended udder. A number of bacteria, mycoplasmas, yeasts and fungi can cause clinical mastitis.
Sub-clinical Mastitis
This type of mastitis does not cause obvious visible changes to the milk, but there may be some chronic inflammatory changes to the udder detected as hardness or ‘knotty’ tissue on palpation of the affected quarter(s). If tested with a California Mastitis Test (CMT) there is normally a reaction due to the increased SCC of milk produced by cows with chronic subclinical mastitis. Several pathogens are associated with this type of mastitis, especially S. aureus and S. uberis. This type of mastitis is important as cows can be a reservoir of infection for the rest of the herd, with pathogens spread during milking. Additionally, the milk may have changes in the amounts of milk solids (protein and fat) present as well as a high SCC count which, if present in a high proportion of cows in the herd may affect the bulk tank milk composition sufficiently to affect the price paid. Lastly, and importantly, these cases may flare-up periodically and cause clinical disease with associated painful symptoms for the cow, which is a concern regarding animal welfare.
Californian Mastitis Test (CMT)
- This is a kit that will help the milker evaluate somatic cell counts in milk from different udder quarters immediately
- It will help the herdsman to decide when to put the milk back into the bulk tank, in order to avoid high bulk tank somatic cell counts and putting mastitic milk into the supply chain
- It will also help the herdsman to recognise affected quarters when the obvious symptoms are mild or he can only see a “clot in the filter”
- It is easy and quick to use, reliable and relatively cheap for what it does
- The reagent must not be allowed to freeze and needs to be warmed up in hot water if it is very cold
- You can get a starter kit from most vet practices or dairy suppliers
Summer Mastitis
Classically, this type of mastitis is seen in dry cows in the late summer period and is associated with a spread of infectious mastitis-causing organisms by flies or by teat damage to dry cows. It can occur also in maiden heifers. This type of mastitis is mainly due to a combined effect of several bacteria. Arcanobacterium pyogenes is the primary organism, but the severity of clinical signs depends also on the other, often anaerobic bacteria present which can include Peptococcus indolicus, Bacteroides melaninogenicus and Fusobacterium necrophorum and their production of endotoxin (Hillerton, 2004). The clinical signs vary and in the early stages may not be seen as cows are not as easily observed when dry as when they are going through a milking parlour. However, the infection will spread rapidly and can cause severe swelling of the udder with a foul-smelling discharge or secretion from the affected quarter(s). The cow may become very dull and depressed, especially if there is production of endotoxin and may develop a very high temperature. Severely affected cows may abort. Cows which are very dull and/or abort should be examined by a vet. Often, the cow or heifer will end up with a permanently non-functional (blind) quarter due to the severity of the inflammation and damage that occurs.
Control and Prevention of Mastitis
As described, mastitis is a multifactorial disease, closely related to the production system and environment in which the cows are kept. Control of mastitis in the dairy herd must take a holistic approach which encompasses:
- Mastitis detection and treatment
- Milking practices
- The cows’ environment (both housed and at grass)
- Feeding practices
- Accurate record keeping
- Regular review of the mastitis records, preferably with the farm vet
As mastitis is one of the most important diseases in dairy farming, prevention and control of mastitis should be a priority on all dairy farms. Thus, all of these components in mastitis control should be integrated in the Herd Health Plan for the farm.
Mastitis – Causative and Risk Factors
Our understanding of mastitis has developed in several stages over the past 100 years. An association between mastitis and pathogenic micro-organisms was established in 1887 (Munch-Petersen, 1938). Most major pathogens were identified by the 1940s. When antimicrobial therapy became available for production animals in 1945 it proved effective in the control of some, but not all, mastitis pathogens (Downham and Christie, 1946). This prompted further research into potential husbandry related causes of mastitis. In the 1960s, the multifactorial aetiology of bovine mastitis was commonly recognized (Neave et al., 1969).
Today, mastitis is considered to be a multifactorial disease, closely related to the production system and environment that the cows are kept in. Mastitis risk factors or disease determinants can be classified into three groups:
Mastitis Pathogens
Over 100 different micro-organisms have been identified as causative agents of mastitis. The importance of the various mastitis pathogens has markedly changed throughout the past 50 years as a result of different control and husbandry methods used. The routine use of antibiotics and improved understanding of the complex aetiology of mastitis have meant that the targeting of control, and even eradication, of some mastitis pathogens, has become more efficient. Increased emphasis on SCC reduction and targeting certain contagious micro-organisms (i.e., S. agalactiae) may have changed the relative importance of the principal mastitis pathogens in the national herd. Clinical mastitis caused by Gram-negative pathogens, such as E. coli, Klebsiella spp., or Pseudomonas spp., occurred more often in herds with a low bulk milk SCC. Clinical mastitis caused by S. aureus, S. dysgalactiae, and S. agalactiae occurred more often in herds with a high bulk milk SCC (Barkema et al., 1998). This phenomenon is coupled with apparent changes in the virulence of some pathogens such as S. uberis (Myllys et al., 1994; Watt, 1997).
There is also increasing evidence that bacteria which until recently have been considered non-pathogenic or opportunistic udder pathogens are becoming more common as primary mastitis pathogens. These bacteria include Corynebacterium bovis and coagulase negative staphylococci (CNS).
Major mastitis pathogens are classified as being either contagious or environmental. Environmental mastitis problems often present as increased number of cases of clinical mastitis within a herd rather than increased BMSCC (Ruegg, 2012).

Contagious mastitis pathogens are usually spread from cow to cow, with the biggest risk period being during milking. Transmission can usually be controlled by good milking hygiene and biocontamination of known cases.
Contagious Mastitis Pathogens
The contagious pathogens usually have a mechanism to adhere to the epithelial cells of the udder or to become intracellular, in order to protect themselves from the intramammary defence mechanisms. S.aureus, S. agalactiae and S. dysgalactiae belong to this group of pathogens. Some S. aureus strains also have the ability to remain intra-cellular and avoid both host natural defences and antimicrobials and may additionally produce β-lactamase which inactivates penicillin. Arcanobacterium pyogenes is an opportunistic, contagious mastitis pathogen. Mastitis caused by these microbes is often chronic and causes elevated SCC levels. These pathogens are primarily spread within the herd from cow to cow, with the biggest risk period being during milking, where bacteria can adhere to milking equipment or the milker’s hands. It is possible to control and eradicate contagious mastitis pathogens from a herd by aggressive antimicrobial therapy and/or culling (Pyorala, 1995), and good biosecurity and milking hygiene.
Environmental Mastitis Pathogens
A linear relationship between the rate of clinical mastitis and the number of Gram-negative bacteria in bedding has been demonstrated (Hogan et al., 1989). Bedding, moisture, mud and manure are common reservoirs for environmental mastitis pathogens, although these pathogens tend to be less adapted to survival in the udder (Ruegg, 2012). In Europe and North America environmental mastitis bacteria include a large number of both Gram-positive ( S. uberis, S, equinus, Enterococcus faecalis and Enterococcus faecium) and Gram-negative (E. coli, Klebsiella spp., Enterobacter spp. and Pseudomonas spp.) species. Other Gram-negative bacteria often isolated from intramammary infections among herds of North America include species of Serratia, and Proteus (Hogan and Smith, 2012).
The significance of S. uberis and E. coli has grown in the past 15 years as S. agalactiae and S. aureus have been controlled successfully in many herds. It has also been suggested by various surveys coliform infections have become more significant and severe in herds with low SCC (Green et al., 1996; Peeler et al., 1994; Schukken et al., 1990; Waage et al., 1998).
Whilst it has been recognised that teat injuries, wet bedding and contamination with faecal material are important risk factors for E.coli mastitis, it has also been demonstrated that improvements in hygiene alone do not reduce E. coli mastitis incidence, as cows are becoming more susceptible to the disease (Bradley and Green, 1998). One of the reasons for the increased susceptibility is likely to be the increased milk flow capacity in cows, leading to milk leaking (Schukken et al., 1990). Also as their milk producing ability increases, the high yielding dairy cows become more susceptible to infectious diseases (such as mastitis) and metabolic stress (Trevsi, 2014). There is also evidence that opportunistic mastitis bacteria, especially Corynebacterium bovis, have a protective effect against major pathogens (Huxley et al. 2003).
Mastitis Opportunistic Udder Pathogens
Opinions on the significance of Corynebacterium bovis as an udder pathogen vary greatly in the literature. Some researchers suggest that whilst intramammary infections with C. bovis cause increased SCC in affected quarters, the presence of this minor pathogen provides protection against major pathogens (Green et al., 2005; Schukken et al., 1990). Others, however, find no protective effect (Hogan et al., 1988) with some work suggesting an increased risk of infection with S. uberis or a coliform in a quarter infected with Corynebacterium spp or coagulase negative staphylococci (CNS) at drying off (Berry and Hillerton, 2002).
In many countries with intensive dairy production, CNS has been identified as emerging mastitis pathogens, suggesting that increasing numbers of bacteria considered non-pathogenic, until recently, are capable of causing clinical intramammary infections (Myllys et al., 1994). Generally, it is accepted that the mastitis caused by these organisms is mild or subclinical. It has been suggested that the susceptibility of dairy cows to mastitis caused by CNS is a reflection of lowered resistance in the cow’s udder (Myllys et al., 1994).
Host Determinants for Mastitis
Genetic Resistance and Mastitis
Work by Pösö and Mäntysaari established a favourable genetic correlation between low SCC and mastitis incidence at the cow level (Pösö and Mäntysaari, 1996). Others have reported on the significance of certain BoLA alleles in resistance to Staphylococcus aureus infection (Aarestrup et al., 1995). A particular concern is that factors increasing resistance to one udder pathogen might predispose to mastitis caused by other pathogens.
In the past, efforts to introduce mastitis resistance traits into dairy cow breeding schemes have been hampered by the negative genetic correlation with increased milk yield. It has often been considered uneconomic to attempt to improve both mastitis resistance and milk yield simultaneously, particularly since the heritability of milk yield is markedly higher than that of mastitis resistance (Strandberg and Shook, 1989). Some Scandinavian countries have included clinical mastitis in bull proofs, and mastitis rates have fallen in the last decades.
Cow and Udder Conformation
Udder and foot conformation have been shown to be important risk factors for mastitis. For instance new intramammary infections have been linked to higher milk flow rates. Udder and teat depths are associated with udder health but the exact relationships are not clear because many other factors such as breed, milking technique, and mastitis treatments vary (Grindal and Hillerton, 1991; Seykora and McDaniel, 1985). Most conformation related traits have high heritability, and are generally recognised by farmers and herdsmen as major selection criteria for breeding. There is an emphasis on appropriate breeding in sustainable farming, and hence breed may be an important factor influencing both mastitis resistance and susceptibility characteristics.
Cow-related risk factors for mastitis include udder conformation, milk yield, nutritional status, age, stage of lactation and SCC history.
Milk Yield
There is substantial evidence to suggest that high yields are linked to high mastitis levels (Chassagne et al., 1997; Gröhn et al., 1990; Rajala and Gröhn, 1998) although this relationship is complex and inter-relates genetics, feeding and management.
Nutritional Status
Various nutritional factors may negatively affect the disease resistance of a cow. The phagocytic activity of macrophages in milk may be significantly inhibited by ketone bodies (Leslie et al., 2000). Energy, protein and mineral/trace element deficiencies may also affect disease resistance and SCC levels. Selenium supplementation has been linked to improved immunity to mastitis (Conrad and Smith, 1986; Erskine et al., 1987; Weiss et al., 1990).
Mastitis and the Age of Host
Both mastitis incidence and SCC levels are higher in older cows (Bendixen and Astrand, 1989; Busato et al., 2000; Emanuelson and Persson, 1984; Harmon, 1994). The excessive culling of young animals could be considered as contradictory to positive welfare management as well as being costly both economically and environmentally.
Mastitis and the Stage of Lactation and Dry Cow Treatments
Most mastitis surveys show that 2/3 of all clinical mastitis cases occur in early lactation (Faye and Fayet, 1986; Jones and Ward, 1989). The dry period is a major risk factor for new intra-mammary infections which may either cause clinical mastitis in the dry period, or, may remain as chronic infections which are seen clinically in the early part of the next lactation (Bradley and Green, 2000). Thus, management of the cow, particularly with respect to the environmental conditions during the dry period can have potentially profound effects on the mastitis incidence in lactation.

Non antibiotic teat sealants will provide protection against new infections, but they will not treat existing ones so they should only be used on cows who do not have a current infection at drying off.
There are non-antibiotic internal teat sealants (ITS) based on an inert bismuth compound available in the UK and the US. This provides farmers with an option for a non-antibiotic preventive approach to dry-cow mastitis. Clinical trials suggest that the product is extremely effective (as good as or better than antibiotics) at preventing new infections in uninfected glands (Huxley et al., 2002). However, the key is to identify those uninfected cows which are most likely to benefit from an ITS. Work has shown that in organic farms with a particular environmental mastitis challenge, an ITS can be very useful in preventing new infections (Klocke et al., 2006).
Mastitis and Other Diseases
It is recognised that other diseases, particularly ketosis, milk fever, lameness and post-puerperal endometritis/metritis, are closely associated with mastitis incidence (Gröhn et al., 1989; Oltenacu et al., 1988; Peeler et al., 1994). The aim in all herds should be to reduce periparturient and metabolic disease as these are two well defined risk factors for mastitis.
Mastitis and the Vaccination Status of Host
A mastitis vaccine covering E. coli and S. Aureus is available on the UK market (Startvac, Hipra). In spite of this, emphasis should be placed on management and husbandry factors that reduce the risk of E. coli infection and not necessarily on the routine use of such a vaccine. It can, however, be used as a part of a disease reduction strategy alongside other management and husbandry improvements in herds where E. coli has been identified as a major problem. The evidence points more towards a reduction in severity than a reduction in mastitis incidence, so its use should be carefully discussed with the vet.
Environmental and Management Determinants of Mastitis
Increased opportunity for growth of pathogenic udder microbes in the cow’s environment

Straw tends to have the highest streptococcal counts, while sawdust has the highest coliform counts, and washed sand consistently contains 100-fold fewer mastitis pathogens per gram of bedding. However, regardless of the bedding used, removing wet, soiled material from the back third of stalls will significantly reduce the bacterial counts (Hogan and Smith, 2012)
A UK study found mastitis incidence in UK dairy herds is higher in those housed in straw yards compared to those housed in cubicles. Nevertheless, the best straw-yarded herds did better than the worst cubicle-housed herds, so that it must be concluded that the yard system can be made to work satisfactorily (Whitaker et al., 2004). As bacterial infection from the environment is involved, clean, dry bedding, good ventilation, regular mucking out, and keeping the cows out of lying areas for 30 minutes after milking are all important factors in helping to prevent mastitis (Whitaker et al., 2000).
In studies carried out in the US that look at bacterial counts of different bedding materials in dairy systems, sand has been shown to have the lowest bacterial counts compared to box compost, horse manure and foam mattresses (van Gastelen et al., 2011), and digested manure solids had the highest number of K. pneumonia compared to recycled sand and wood shavings, and clean sand had the lowest (Godden et al., 2008). In the same study wood shavings harboured the lowest number of E. faecium compared to the other bedding materials (Godden et al., 2008), although sawdust and wood shavings are often associated with higher numbers of K. pneumonia (Newman and Kowalski, 1973). In Germany a team of researchers compared bacterial counts on teat skin and canals of cattle bedded on sawdust treated with an alkaline conditioner (hydrated lime) versus untreated. They found that the teat skin and canals of cattle bedded on the treated sawdust had significantly lower bacterial counts for S. aureus, S. uberis, E. coli, and coliform bacteria (Paduch et al., 2013).
Mastitis and the Introduction of New Pathogens into the Herd
The most likely risk factor for introducing new contagious mastitis pathogens into a dairy herd is a new cow or heifer that carries an infection. A closed herd policy is the best safeguard against this.
Mastitis and Culling Policies
The presence of chronically infected cows in a dairy herd is a well-recognised risk factor for mastitis. Due to poor cure rates with antibiotic treatment and due to their contagious nature, S. aureus infections are seen as particularly dangerous and culling may be the most effective means of control in some cases.
Mastitis Treatment Practices
Mastitis treatment practices can affect the transmission of pathogens within the herd. If the main aim of the treatment is not to eliminate the pathogen as quickly as possible, the duration of infection increases, increasing the risk of transmission.

Milking hygiene is the basis for control of contagious mastitis but has less influence on environmental mastitis pathogens.
Milking Hygiene
The main aim of milking hygiene is to prevent the spread of contagious mastitis from one cow to another and to prevent the introduction of environmental or contagious bacteria inside the teat canal during milking. The most effective ways of avoiding these risks is to milk infected cows separately or last and to keep udders, teats and the milking machine clean.
Mastitis and Udder Cleanliness
Udder cleanliness is an important factor in the general prevention of mastitis. Dirt on udders and teats increases infection pressure, damages skin and prevents beneficial, commensal flora from establishing. As cows become dirtier in both organic and conventional systems, it has been shown that the herd BTSCC increases, suggesting an increase in mastitis (Ellis et al., 2007). Additionally, cows housed in straw yards have been found to be more likely to be dirtier than those housed in cubicles (Ellis et al., 2007).
Mastitis and Disinfection of Teats Before and/or after Milking
Most germicidal teat dips effectively destroy microbes on the teat skin by chemical or biological action, however their persistence is limited and can be neutralised by organic materials such as milk or manure (Hogan and Smith, 2012).
Teat disinfection after milking has a major effect on the microbes growing on the teat that may have been spread during milking, although the effectiveness is dependent on the time of application relative to milking and the pathogens causing mastitis (Hogan and Smith, 2012). Disinfecting of teats before milking is recommended as a way of reducing new intramammary infections caused by environmental pathogens during lactation (Hogan and Smith, 2012). Whilst post milking teat disinfection has been very successful in combating contagious mastitis, it has been suggested that, as well as killing off the pathogenic microbes, the disinfectants also destroy other microbial flora that function as “healthy” competition against colonisation of the teat, particularly by environmental mastitis-causing organisms (Green et al., 1996) although this hypothesis is not clear and current good practice would still be to disinfect after milking. However, some herds with very low cell counts and severe toxic mastitis have ceased post milking teat disinfection in order to preserve opportunistic udder bacteria such as C. bovis. Teat disinfection is one of the easiest and most comprehensive ways of reducing mastitis spread within a herd and can therefore reduce the subsequent antimicrobial use. Also, the pre-milking teat disinfection routine if carried out at the correct time relative to cluster attachment can help stimulate milk let down via oxytocin release which may help avoid over milking and subsequent teat end damage.
The current recommendations in North America for pre-dipping include:
- Forestripping the first few streams of milk
- Removing excess manure and dirt from teats,
- Dipping teats in germicidal teat dip, allowing teat dip to contact teat skin for at least 30 seconds
- Manually drying teats with individual paper towels (Hogan and Smith, 2012)
Products for pre-milking disinfection require fast kill rates but no teat conditioners, while products for post milking disinfection can tolerate slow kill rates but require persistency and must contain emollients to condition the teat skin. Therefore, pre- and post milking disinfection is usually done with different products.
Mastitis, the Milking Machine and Milking Technique
Milking machine faults and poor milking techniques are probably among the main risk factors for mastitis, alongside housing hygiene. Unstable or excessive vacuum, faulty pulsation, liner slippage for various reasons and teat cup hygiene contribute to mastitis risk by either damaging the teat canal or by causing pathogenic organisms to enter the teat canal during milking (Manninen, 1995). The degree of attention paid to milking machine maintenance can vary between farms. This is an area which should be highlighted in the herd health plan as part of the control measure against mastitis.
The teat canal remains open for up to 45 minutes after milking. A recommended husbandry practice is to prevent the cows from lying down until the teat canal has closed, to prevent bacterial penetration.
Mastitis and Teat Injuries
Teat injuries are associated with increased risk of mastitis reported in cows with teat lesions (Lewis et al., 2000). The main causes of teat injuries tend to be due to inappropriate housing systems as well as poor milking machine performance which can damage teat ends and lead to hyperkeratosis.
Stockmanship
Stockmanship and other characteristics of the herdsman have been considered important enough by some researchers to warrant a separate category among disease determinants (Schwabe, 1984). There is, however, very little published information on direct correlations between mastitis and stockmanship.
The Conventional Approach to Mastitis Control and Prevention
For the past 30 years, the theory and practice of conventional mastitis control in the UK have has been based on the Five Point Plan, developed at the National Institute for Research in Dairying in conjunction with the Central Veterinary Laboratory, in the 1960s. Historically it was targeted against contagious mastitis which was the predominant problem at the time. Therefore it did not cover environmental management or pre milking teat disinfection.
Parts of the ‘Five Point Plan for Control Of Mastitis in Dairy Herds’ are still relevant but time have moved on in many aspects:
- Routine post milking teat dipping or spraying is still recommended on most dairies except those with very low cell counts and a problem with severe environmental mastitis (see above)
- The prompt treatment of clinical mastitis with antibiotics is still important, however, a more selective approach is increasingly adopted in North America and Europe (see below). This is based on different self cure rates of different pathogens and the availability of on farm test kits.
- Blanket dry cow therapy with antibiotics can no longer be recommended (see above)
- Culling of cows with chronic mastitis is still relevant
- Regular milking machine testing and maintenance is still relevant
The Sustainable Approach to Mastitis Control and Prevention
Sustainable livestock principles promote positive health care and prevention of diseases, particularly by improving or supporting an animal’s own defence mechanisms. Good husbandry, breeding of resistant animals and optimisation of production levels are seen as the cornerstones of mastitis prevention.
Treating Mastitis
A healthy bovine udder should have the capacity to clear itself of infection without therapy. In the case of some pathogens, like E. coli, the self-cure rates can be as high as 90% (Sandholm, 1995a). The purpose of mastitis therapy is to assist the affected quarter to clear infection as rapidly as possible and to enable a quick return to normal milk production. The therapy should also be directed to alleviating pain and discomfort caused by inflammation associated with mastitis except in cases of toxic mastitis where treatment is an emergency and should be undertaken by a vet immediately.
Toxic Mastitis
Cows with toxic mastitis are often in, or at risk of endotoxic shock. Therefore treatment should include administration of supportive therapies. The cow may require administration of fluids, and the use of hypertonic saline intra-venously can be effective in increasing the circulating blood volume, although it is imperative that the cow either drinks water herself or is given water (30- 50 litres depending on size of cow) by stomach tube following use of hypertonic saline. Additionally, the use of non-steroidal anti-inflammatories (like flunixin meglumine) can relieve pain and helps reduce the pyrexia if present and can help combat the endotoxins. Administration of antimicrobials is also advised as the cow’s immune system will be severely compromised at this time. Above all, cows should receive plenty of nursing, including frequent turning if recumbent and frequent stripping out of the affected quarter(s) to help remove the altered milk which will contain endotoxin (Sandholm, 1995b). However, many cases of toxic mastitis are unresponsive to treatment.
Mastitis Therapy in Mild to Moderate Cases
Prompt treatment is encouraged on animal welfare grounds. The use of alternatives to antimicrobials is encouraged ‘where effective’. However, there is considerable debate over the effectiveness of other alternative remedies and the use of unlicensed products in food producing species is an area of concern.
Intramammary application of antibiotics is the most common form of therapy. It is evident that the use of bacteriological examination to identify the mastitis pathogen or to target the antibiotic therapy is not common. Hovi and Roderick (1999) found that 68.5 % 18.5% of all antibiotic treatments on conventional and organic farms respectively were in excess of the manufacturer’s recommendations (Hovi and Roderick, 1996).
Alternative therapies adopted by farmers include:
- Frequent stripping out
- Cold hosing
- Application of udder creams and ointments, for example Uddermint™ and Golden Udder™.
Antibiotic Therapy
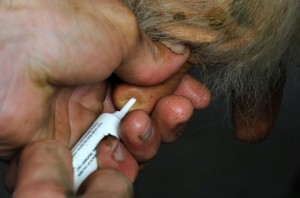
Antibiotic dry cow therapy should be carried out on a selective case by case basis and not as a blanket prophylactic herd level treatment regime.
The emphasis of clinical mastitis treatment has been on antimicrobial therapy since antibiotics were first introduced to production animal medicine in the 1940s (Bramley and Dodd, 1984). The advantages of antibiotic mastitis therapy are the potential high cure rates when the treatment is well targeted and ease of use that suits modern dairy management systems. The major disadvantages are potential residues in milk, the development of antimicrobial resistance, disruption of symbiotic gut flora of the host when systemic administration is used and interference with phagocytic activity in the udder when lipid soluble intramammary preparations are used (Erskine et al., 1987; Paape et al., 1990; Sandholm, 1995a).
Antibiotic therapy should be encouraged in the treatment of mastitis caused by Streptococcus agalactiae as this pathogen is zoonotic and is easily eradicated from a herd. When this pathogen is identified a vet should be contacted and an eradication plan should be implemented.
Alternative Therapies
A multitude of mastitis therapies have been used before and after the advent of antibiotic therapy. These include the use of frequent stripping, herbal udder ointments and liniments, massage, phytotherapy, diet changes, clay therapy and homeopathy (Duval, 1995). The use of oxytocin combined with frequent stripping is a more “modern” alternative therapy (Knight et al., 1997). Most alternative therapies are aimed at helping the cow’s own defence mechanisms to clear the infection rather than attacking the pathogen directly as is the case with antibiotics.
The main advantages of these therapies are that they work together with the cow’s own defence mechanisms to clear the infection and they often do not have long withdrawal periods. However, many of these therapies are time-consuming, labour intensive and often require separation of the treated animal from rest of the herd. They are consequently poorly suited for modern cow housing and milking systems with loose housing and rapid throughput parlours. Since very few are classified as licensed medicines, there is no requirement for clinical testing. Consequently, there is little information regarding their efficacy. The risk of fraudulent or ineffective products being marketed also increases in the absence of statutory monitoring. Farmers should be particularly careful not to use preparations that are not licensed for intramammary use, as these can either cause damage to the udder or lead to residues in milk.
Homeopathy and Mastitis: Prevention and therapy
Homeopathy is widely used in the UK and in Europe, alone, or as an adjunct to other therapies (Ellis, 2005; Hovi and Roderick, 1996) and is recommended for both mastitis prevention and treatment (Hansford and Pinkus, 1998; MacLeod, 1991). There is, however, very little evidence of the efficacy of homeopathy in either curing or preventing mastitis in dairy cows. Whilst a substantial body of anecdotal evidence about successful mastitis therapy or prevention exists (Martini et al., 2001; Merck et al., 1989; Tiefenthaler, 1994, 1995), several review papers and published experimental work have all drawn the conclusion that homeopathy has no beneficial effect or that its efficacy has not been proven (Hamann, 1993; Hektoen et al., 2004; Meany, 1995; Schutte, 1994; Stopes and Woodward, 1990; Turner, 2001). In particular homoeopathy has been reported to have a poor effect in subclinical mastitis (Walkenhorst et al., 2001) and S. aureus treatment (Tikofsky and Zadoks, 2005), although treatment effects may be seen in cases of acute mastitis as the cow has a better chance to react against infection compared to a cow with chronic mastitis (Walkenhorst et al., 2001). The apparent lack of effect of homoeopathy may also be a reflection of the complex nature of homeopathic prescriptions and the lack of expertise among both the users and the researchers (Hektoen et al., 2004). The apparent high cure rates against clinical mastitis on some farms may reflect the high self cure rates of coliform mastitis.
A detailed description of the principles of homoeopathy as well as other alternative therapies, including suggested approaches to utilising alternative therapies in cattle is given by Day, 2004.
In the light of this lack of understanding of the usefulness of homeopathy and the wide-spread use of homeopathy among farmers, the importance of good recording of all treatments, including the outcomes of the treatments, on farms that use homeopathy should be emphasised.
Mastitis Therapy and Pain Relief
An important aspect of mastitis therapy is the alleviation of inflammation in the udder. Swelling and subsequent pain associated with clinical mastitis can cause considerable discomfort to the cow. In the light of recent research findings on subjective pain measurement in mastitic cows, pain relief should be an important objective of mastitis therapy (Barrett, 2004; Fitzpatrick et al., 1998).
Antibiotic preparations offer no immediate relief from pain. Whilst the inclusion of steroid anti-inflammatory components in intramammary preparations has very little effect on the inflammation, systemic use of non-steroidal anti-inflammatory agents, can have a beneficial effect on the inflammatory process in acute mastitis (Lohuis et al., 1990). There are a number of non-steroidal anti-inflammatory drugs (NSAIDS) now licensed for cattle and their use should be encouraged to relive pain associated with mastitis. However, some products have a relatively short duration of action (Fitzpatrick et al., 1998), while others have long acting efficacy. The choice of product should be discussed with a vet. Use of oxytocin and frequent stripping is likely to alleviate some pain by relieving the tension in the affected quarter, but no published information on its efficacy is available. Similarly, many alternative therapy forms, including udder liniments, clay therapy and cold water massage, are likely to alleviate pain by increasing circulation and reducing swelling. A recent small study in the UK found that Uddermint routinely given at calving reduced cell counts in the lactation.
Selective Antibiotic Treatment for Clinical Mastitis
Based on the high self cure rates of mild and moderate coliform (Gram-negative) clinical mastitis cases, on farm culture tests are available which enable farmers to treat Gram-positive cases only. Lago et al. (2011a, 2011b) found that this approach led to a 50% reduction in antimicrobial use for clinical mastitis without affecting cure rates, recurrence rates and milk quality parameters. More on farm tests are becoming available in different countries and this is a promising approach, based on good evidence to significantly reduce antimicrobial use.
Prevention and Treatment of Mastitis in Dry Cows
Dry cow hygiene (adequate stocking rate, clean and dry bedding, good ventilation) is an area many farmers can improve on. The record analysis on cell counts and clinical mastitis can show whether the dry period is a major problem.
Selection of appropriate therapy to dry cow mastitis treatment and prevention is an area where the vet can help the farmer as part of the herd health plan to determine which cows should receive antibiotic DCT (in combination with an internal teat sealant) and which cows should receive an internal teat sealant only. This decision should be based on cell count and clinical mastitis history of the cow.
Routine DCT of all cows, independent of their udder health status, has been criticized for potentially contributing to the development of antimicrobial resistance on farms (Huda et al., 1997). However, the efficacy of antibiotic DCT in mastitis therapy, curing chronic infections and protecting against new infections during the dry period, is widely recognised (Ziv et al., 1987). The apparent control of contagious pathogens like S. agalactiae and S. aureus in England and Wales has been attributed to the “blanket” antibiotic use at drying-off (Booth, 1997).
Using an internal teat sealant (ITS) can provide protection against new infections in the dry period but it is important to emphasise that this will not specifically ‘treat’ existing infections. Selection of cows for use of ITS is therefore important, as only those cows with no current infection at drying off should be considered. It is recommended that cows with a cell count of consistently <200,000 cells/ml at drying off and no previous recent cases of clinical mastitis should be selected. However, these criteria are difficult to meet in the field and a modification of the selection criteria, based on the knowledge of the individual farm can be utilised (see Practical Advice at the end of this chapter).
The use of antibiotic DCT for the minority of individual cows for treatment rather than prevention can be justified on a selective basis and as a part of planned mastitis reduction strategy.
Mastitis and Welfare
It has been commonly recognised that mastitis is a painful condition, and that one of the aims of mastitis therapy should be pain relief (Fitzpatrick et al., 1998). Early and efficient treatment of clinical cases of mastitis should be a requirement for dairy farms to be able to claim high welfare status.
Good Practice Based on Current Knowledge
Mastitis is an extremely important disease and therefore should be a priority consideration in the herd health plan of ALL dairy farms.
Create a Mastitis Control Strategy
It is important to consider each farm on an individual basis and to design mastitis control strategies and set targets that take into consideration the unique features of each farm, rather than expecting all farms to achieve a common standard.
1. Creating and maintaining mastitis monitoring systems
The mastitis records should include the following:
- Name/number of the cow
- Affected quarter/quarters
- Dates, duration
- Frequency and type of treatment
- Length of the statutory withdrawal period
- Outcome of the treatment (e.g. success/failure/lost quarter/cull/removal to suckling etc.).
How to interpret your mastitis records
The key mastitis parameters are:
- Number of cases/100 cows (no. of cases recorded divided by no. of milking cows multiplied by 100): if this figure is above 35 cases/100 cows or if it is higher than during the previous observation period, the farm should take action to improve the situation
- Proportion of the herd affected (no. of cows with at least one case of mastitis in the observation period divided by no. of milking cows): if this figure is high, you are probably dealing with an environmental mastitis problem; the estimated national average is around 25%
- Recurrence rate (no. of quarters that have had one or more cases of mastitis in the observation period divided by no. of all affected quarters multiplied by 100): if this figure is high, your treatments are probably not very effective and/or you are dealing with chronic infections that are difficult to cure; the national estimate is around 18%
2. Find out what is causing mastitis on your farm
For a good targeting of mastitis control measures, it is important to know what is causing mastitis in a herd. The best starting point is to contact the vet for advice on where to have the bacteriological examinations done.
If the farm is not a member of a milk recording scheme, they should consider starting monthly milk recording that includes SCC measurements, and learn to use the Californian Mastitis Test (CMT)
It is important to keep an eye on the SCC throughout the lactation to be able to identify subclinically infected cows. Decision-making about drying off and culling should be based on SCC recordings and bacteriological examination.
CMT will help the herdsman to monitor the situation in between recordings and to identify infected quarters. It is also helpful in determining when to include milk in the bulk tank after a case of clinical mastitis. A system to analyse mastitis records (both treatments and SCC) every three months should be set up to find out what the mastitis levels on the farm are like.
Bacteriological examination of milk samples
- Taking samples from all new cases of mastitis is good practice and can help to identify the epidemiology of infection in a herd and therefore can help in targeting the most effective control measures (Biggs, 2003; Bradley et al., 2002a)
- The farm vet might be able to carry out the bacteriological work. If not, she or he will be able to advise and arrange sending samples to a commercial laboratory or VI centre that will do it
- Often, the cheapest way to get bacteriological examinations done on milk samples is to freeze the samples over a period of time and send a number of them to a laboratory at the same time (be sure to agree with the laboratory that they will accept frozen samples beforehand)
- Some commercial laboratories offer considerable savings when multiple samples are submitted
- Taking good milk samples for bacteriology requires skilled practice and great care. Advice should be sought, from the farm vet
3. Minimise the presence of contagious and environmental mastitis pathogens in the herd
Create a herd that enhances good udder health:
- Breed heifer calves from healthy cows with good longevity and conformation
- Maintain good foot health and a closed herd policy
- Keep your herd free of BVD (Bovine Viral Diarrhoea) and other contagious diseases that may impair immune function
- Keep the cows in stable groups
- Keep a separate heifer group for introduction into the milking herd
- Minimise the energy deficit in early lactation
- Separate infected and high SCC cows into their own milking group and milk last
- Cull chronically infected cows
- Keep the milking machine serviced
- Use scrupulous milking hygiene
- Both pre- and post-dipping the teats
- Disinfecting the clusters after high SCC cows or use a back flush system if contagious mastitis is a problem
Keep the housing conditions as clean and dry as possible:
- Do not use wet bedding
- Re-bed as often as you can – Straw yards must be bedded daily and cleaned out monthly
- Respond to changes in weather by increasing bedding when very wet
- Scrape the floors at least twice a day
- Avoid water troughs in the bedding area
- Train heifers to use the cubicles correctly
- Ensure there are enough cubicles for the number of cows (a 5% excess is recommended to optimise cubicle use)
- Assess the cleanliness of your cows regularly during the housing period – if they are dirty, find out why
- Do not let the cows access bedding until 30 minutes after milking
Keeping SCCs low:
- Maintain a system of a monthly individual cow SCC recording to identify chronically high SCC cows and to treat or cull them in accordance with advice from your vet
- Withdraw the milk long enough after a case of clinical mastitis, even when alternative medication with no withdrawal period is used
- Use the Californian Mastitis Test to determine when the milk is ready to go back to the tank or use a quarter milker
- Put high SCC cows to be suckled by calves until SCC levels reduced (test with CMT )
- If your BTSCC increases find out the cause for the high levels
Practice a closed herd policy but if you have to buy in replacements:
- Check individual cow cell counts records if available
- Isolate them for a minimum of 21 days
- Sample all high cell count quarters for bacteriology
- Include in the main herd first when udder health status clear
Identify and treat any cases of clinical mastitis promptly and with means that will also alleviate the pain associated with clinical mastitis:
- Use CMT or other cow side tests to identify suspected cases/quarters immediately
- Consider installing an in-line mastitis detection system if you are renewing your parlour
- Always use anti-inflammatory drugs and consider using udder creams (Uddermint™ or Golden Udder™) or cold water massage (minimum of ten minutes per treatment) and frequent stripping to alleviate the pain of swelling and local symptoms in the udder
4. Drying off cows without blanket antibiotic dry cow therapy
In order to phase out blanket antibiotic dry cow therapy (DCT) gradually farmers will need to dry the cows individually. It is important to take into account the history of each cow on an individual basis, for example:
- Did she have repeated (>5) cases of clinical mastitis?
- Is information available on bacteriological culture from mastitis samples (e.g. has Staph aureus been isolated)?
- Are monthly ICSCC results available and are any of the last three readings >200,000?
A suggested decision-making tree for selective dry cow therapy is outlined by (Bradley et al., 2002a) for conventional herds. Decision-making is farm-specific and there can be some grey areas, however, discussions with the herdsman and the vet should be beneficial.
Other practical options to consider are:
- Improvement of dry cow management, particularly during the first and last two weeks of the dry period when the cows are vulnerable (i.e., clean and dry housing conditions with daily bedding and adequate stocking density, avoid putting recently dried off cows on contaminated paddocks etc.)
- Abrupt drying off is recommended but requires the ability to separate the dried off animal from the sight and sound of the milking herd, on a limited diet
- If cows are still producing a lot of milk when dry-off is approached, consider extending the lactation. However, the dry period should not be shorter than 35 days. All cows producing
- External teat sealants at drying off can be considered to prevent infection (reapply external teat sealants daily if practical). During the summer months tar can routinely be used to repel flies
- Ensure that transition cow management is optimum, so that the ration is formulated to prevent over-fat cows at calving, that milk fever is minimised and peri-parturient metabolic disease risk is reduced
- Ensure that cow calve down in clean calving boxes, or calve in a clean calving paddock outside


 American English
American English


Comments are closed.