Poultry Diseases
Coccidiosis in Poultry
Also known as: Eimeria
Although there may be some differences between poultry management systems regarding the degree of risk from coccidia, it is generally accepted that the disease may be found in most systems, both indoor and outdoor.
Life cycle of Coccidia
The life-cycle is rapid, approximately 4-5 days, and involves massive multiplication from ingestion of a single infective oocyst. Both infected and recovered birds shed oocysts. Under conditions of 25-30˚C, this takes 1-2 days. Sporulated oocysts have the ability to survive outside the host for very long periods. Since sporulation does not occur below 12˚C or above 39˚C, during winter months spores may remain dormant. Sporulation can continue when temperatures increase, although prolonged periods at low temperatures can destroy the viability of the oocytes. This may be an important factor for outdoor poultry systems. From: (Fanatico, 2006)
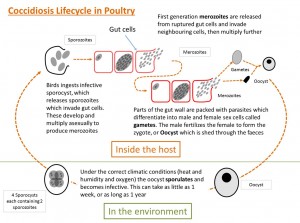
- The life-cycle is short and starts with the bird ingesting sporulated oocysts
- The sporulated oocysts contain four sporocysts, each containing two sporozoites and the mechanical and acidic environment in the gut result in the release of these sporocysts and sporozoites into the gut.
- The sporozoites invade the duodenal mucosa epithelial cells before undergoing phases of growth and multiplication with periodic release of merozoites into the gut.
- Merozoites develop within the duodenal cells as gametes, in the form of both macro- and microgametocytes
- These develop into a zygote and then an oocyst which is shed in the feces
- These oocysts require moist conditions to undergo sporulation, a process that requires oxygen and takes about 24 hours, at which point they become infective.
Symptoms and Pathology of Coccidiosis
Typically, the disease is seen in birds of 3-6 weeks old, before they have acquired immunity. Infected birds will have ruffled feathers, and a breakdown in intestinal function is the first recognizable symptom of coccidiosis, blood in the droppings may be observed depending on the strain of coccidia affecting the bird. The extent of the pathogenicity depends on the species of parasite, numbers of infective (sporulated) oocysts in the environment and the age and susceptibility of the birds. The most virulent strains will cause diarrhea and a sudden increase in flock mortality. Less virulent strains will result in poor growth and reduced feed efficiency. There is normally a reduction in feed and water intake (Williams, 1996). Hence, the losses resulting from coccidiosis are variable.

Most scientific knowledge on coccidiosis has been obtained from chickens as it is in commercial poultry that this parasite causes the most damage due to the fact that birds are reared together in large numbers and high densities (Peek and Landman, 2011).
Coccidiosis rarely occurs in layers and breeders as they have generally acquired immunity to this disease, although in situations where there is an immunity breakdown all pathogenic Eimeria species may cause an abrupt and severe drop in egg production for three to six weeks. Any recovery from severe infection can take 10-14 days, and it takes longer to reach pre-infection production status.
There is likely to be variation within a flock with regard to the response to infection. Within a single infected group there will be individuals dying whilst others will remain healthy. Sub-clinical infection may mean that some birds will take longer to eat, and as a result may become stunted. This will result in uneven groups, which can cause marketing problems in table-bird flocks.
The most common form of the disease is cecal coccidiosis, caused by Eimeria tenella. This normally occurs between 4 and 6 weeks of age. A small but sudden rise in mortality may occur and dead birds will have an anemic appearance. The outbreak tends to occur amongst a single group or house. It is very important to treat when the disease is first seen.
Tissue damage and changes in the intestinal tract, as a consequence of infection, may allow colonization by other harmful bacteria, such as Salmonella typhimurium, or Clostridium perfringens, which causes necrotic enteritis (Van Immerseel et al., 2004).
Coccidiosis may be diagnosed at post-mortem by the presence of characteristic lesions in the intestinal tract, although rapid changes in the intestine more than one hour after death may not allow easy identification. Microscopic examination may reveal the presence of oocysts, but not necessarily indicate clinical disease. Fecal egg counts can be used to detect coccidial oocysts, however high levels may be seen in dung samples without disease being present.
Classification of Eimeria
E. necatrix and E. tenella are the most pathogenic in chickens. Infection with E. tenella can be recognized by blood in droppings and around the cloaca. Other important less pathogenic strains affecting chickens include E. acervulina, E. maxima, E. praecox and E. mitis. The most important species of Eimeria which cause disease in turkeys are E. adenoides and E. meleagrimitis.
The causative organisms are identified and classified by morphological characteristics, their location in the intestines, histological examination of lesions and size of meronts in mucosal smears. These are time consuming and expensive. Coccidial infections can be confirmed by the presence of oocysts in the feces, however, the presence of these can have little or no relationship to an impending or existing infection. Although still necessary, they are now complemented by molecular methods that involve PCR diagnostic assays based on DNA amplification (Chapman, 2014). If a farmer is struggling to control a coccidia, it might be beneficial to a farmer to determine the species of Eimera on the farm to enable a more targeted treatment strategy.
Risk factors for coccidiosis in poultry
Oocysts may remain in buildings from a previous batch of birds, and they may be carried by mechanical means, including equipment, clothing, insects and other animals. Susceptible birds introduced to an contaminated building will quickly become infected. Graat et al. (1998) examined risk factors on 144 broiler farms and found that poor hygiene related to personnel, feeding and drinking was important, as were the presence of other diseases on the farm and if Eimeria species were found in the previous flock.
Whole wheat feeding, compared with a complete ground and pelleted feed, has been shown to increase parasite development during infection with the E. tenella. This might be explained by modifications of digestive physiology and intestinal microflora by whole wheat (Gabriel et al., 2003). However, it is not clear if this is due to poor gut health generally and dietary deficiencies.
Immunity and Coccidiosis in Poultry
Day old chicks do not normally acquire passive immunity from hens, although the potential of maternally transmitted antibodies as a means of control has been investigated (Smith et al., 1994a, 1994b; Wallach et al., 1995). Birds of all ages are susceptible. Chickens can develop immunity after infection, but this immunity is species-specific, leaving birds susceptible to other Eimeria species. Immunity to Eimeria species is acquired gradually and is not complete until the birds are 7 weeks of age – up to this age is therefore the critical period of risk.
Usually immunity will be acquired by a flock by “trickle” infection without the occurrence of clinical disease. However, if environmental conditions, such as wet litter, promote sporulation, birds that have not acquired immunity (typically 3-6 weeks) will succumb to clinical infection.
- Stocking rates – higher enable parasite to transmit between susceptible hosts
- Age of stock – younger birds are more susceptible to disease
- Health status of birds – unhealthy birds have a weaker immune system and are more susceptible to infection
Management-related factors
- Feed – if feed is not palatable or available birds will eat their own feces thus increasing the number of coccidial oocysts they ingest.
- Quality of litter – it must be dry
- Clean out litter between batches – if litter is kept clean and dry and old litter is disposed of after each batch of birds there are less likely to be problems
- Period of barn vacancy – Rotation of pastures – this can depend on the virulence of the strain of coccidia on farm – if it’s a virulent strain the longer pastures and houses can be rested the better – there can be an increase in pathogenicity if there is continued infection of birds from batch to batch
- Sanitation of personnel, vehicles, and buildings
- Insects and vermin – can carry coccidia back into “clean” areas
- Ventilation – poor ventilation leading to high humidity encourages growth of coccidia
- Biosecurity is important for many diseases, but it won’t keep coccidiosis out
Immunosuppressive diseases, such as Marek’s disease, infectious bursal disease (IBD) and others, interfere with the development of immunity, and infected birds can be more susceptible to coccidiosis (Biggs et al., 1968).
Control and Prevention of Coccidiosis in Poultry
It is impossible to completely remove coccidial oocytes from a farm environment. Hence, control should focus on producing environmental conditions that substantially reduce the number of infective oocysts. It has been shown that coccidia oocysts are able to withstand some disinfectants, although they are killed by noxious gases such as ammonia and methyl bromide. However, good husbandry is fundamental in preventing coccidiosis spreading around the farm. Oocysts can be disseminated by feces, litter, invertebrates, vermin, personnel and vehicles.
It is advisable, with good management, to allow some exposure to coccidia so as develop immunity in birds.
Poultry Litter
Keeping litter dry paying special attention to areas near drinkers and feeding troughs can assist in minimising oocyst sporulation. The location of watering facilities in particular can be critical in the control of litter dampness. Feeders and drinkers should be kept at a height that feces cannot contaminate them. Furthermore, since older animals may produce very large numbers of oocytes that may be lethal when ingested by young chicks, it is best to keep age groups separate.
Vaccines for Poultry Coccidiosis
Coccidial vaccines have been used to a limited degree by the poultry industry for about 50 years. Their effectiveness hinges on the recycling of initially very low doses of oocyst, and the gradual build-up of solid immunity (Allen and Fetterer, 2002). They have been used primarily to protect breeder and layer flocks (Shirley et al., 1995). However, their use, particularly in broiler flocks, is increasing (Allen and Fetterer, 2002). There are many different coccidiosis vaccines available, discuss with your vet or other adviser which vaccine and administration route are best for you. Note that vaccines – particularly those that cover many Eimeria species – are difficult to produce and may sometimes be unavailable. In order to achieve sustainable coccidiosis control careful husbandry in combination with vaccination could be considered.
Some producers prefer not to use prophylactic treatments to control coccidiosis in poultry, and to treat animals therapeutically when signs of infection occur. Such a strategy does allow the development of natural immunity, but requires high levels of management and vigilance to avoid serious outbreaks.
Natural Feed Additives
A number of natural products or feedstuffs have been tested as anticoccidial dietary additives. The mounting problem of drug resistance of Eimeria species has prompted major research efforts to seek alternative means of control through increased knowledge of parasite biology, host response, and nutritional modulation. As a consequence, important advancements have been made, particularly in defining parasite antigens that have potential use in vaccines, defining the Eimeria genome, understanding the immunology of coccidial infections, and the practical applications of live vaccines (Allen and Fetterer, 2002).
There have been some very good results with feeding oregano extract or essential oil to chickens (Giannenas et al, 2003, Batungbacal et al, 2007), with evidence that oregano essential oil exerted an anticoccidial effect against E. tenella, with treated chickens showing body weight gains and feed conversion ratios no different to the uninfected control group (Giannenas et al, 2003). However, one study found that oregano may only be effective in birds that were not already vaccinated against coccidia (Oviedo-Rondon et al, 2006).
Treating Coccidiosis
Many products are available for prevention or treatment of coccidiosis in chickens, via both feed and water, with withdrawal periods ranging from 0-10 days. Some drugs can be used to treat the disease and support the build up of immunity, whereas others only treat infection at the time of administration, with follow-up doses required. Coccidiostats suppress the development of the parasite and coccidicidal drugs kill the parasite. Anticoccidials should not be used in laying hens because of possible drug residues in eggs for human consumption.
There is some research to suggest that dried leaves of Artemisia annua, which also has anti-malarial properties, have been shown to have anticoccidial properties against some species of Eimeria when given as an in-feed supplement (Allen et al., 1997). It was been found effective in reducing oocyst output from both E. acervulina and E. tenella infections when fed at levels of 8.5 and 17 ppm in starter diets.
The mode of action is also thought to involve oxidative stress. (Allen and Fetterer, 2002). Efficacy of extracts from 15 different Asian herbs were tested for anticoccidial activity, including Bupleurum chinese DC, Sophora flavescens Aiton, and Artemisia annua. Of these, extract of S. flavescens was the most effective. P. koreana, S. acutum, U. macrocarpa and Q. indica were also effective. (Youn and Noh, 2001).
Good Practice Based on Current Knowledge
Coccidia are almost universally present on any poultry farm, but clinical disease only occurs when susceptible animals ingest a large number of sporulated oocysts, so control should be focussed on preventing these birds from getting clinical coccidiosis.
- Maintain vigilance and treat as soon as the first symptoms are seen
- Keep different age groups separate
- Ensure that litter is dry but not dusty – avoid any causes of wet litter
- Keep litter dry round watering points – do not allow drinkers to overflow
- Ensure high standards of hygiene of personnel
- Ensure good hygiene of feeding and drinking equipment
- Keep raising the level of drinkers as chicks grow to reduce fouling
- Vaccinate if the risks of disease are high


 British English
British English


Comments are closed.