Sheep Diseases
Enzootic Abortion in Sheep
Also known as: Chlamydial abortion, Ovine Enzootic Abortion (OEA), Enzootic Abortion in Ewes (EAE)
 There are many pathogens, including those belonging to the bacteria genera Chlamydia, that impact on the specialized immune systems in the reproductive tract of sheep (Entrican and Wheelhouse, 2006). Chlamydial abortion of ewes is a major cause of reproductive failure and economic loss in sheep production (Nietfeld, 2001; Longbottom et al., 2013). In the United Kingdom, it is the most frequently diagnosed form of infectious abortion in lowland flocks (Leonard et al., 1993; Longbottom et al., 2013) and causes significant losses worldwide. It was first reported in North America in 1958 (Chalmers et al., 1976), and in north central US it has been recorded as being responsible for approximately 5% of abortions in sheep flocks (Dubey and Kirkbride, 1990).
There are many pathogens, including those belonging to the bacteria genera Chlamydia, that impact on the specialized immune systems in the reproductive tract of sheep (Entrican and Wheelhouse, 2006). Chlamydial abortion of ewes is a major cause of reproductive failure and economic loss in sheep production (Nietfeld, 2001; Longbottom et al., 2013). In the United Kingdom, it is the most frequently diagnosed form of infectious abortion in lowland flocks (Leonard et al., 1993; Longbottom et al., 2013) and causes significant losses worldwide. It was first reported in North America in 1958 (Chalmers et al., 1976), and in north central US it has been recorded as being responsible for approximately 5% of abortions in sheep flocks (Dubey and Kirkbride, 1990).
Chlamydophila abortus (Chlamydia psittaci), does not generally cause clinical signs in the ewe other than abortion. There is visible damage to the placenta, which may be inflamed and thickened (Buxton et al., 2002). Abortion is the result of several factors, including destruction of tissue by C. abortus, vascular thrombosis, and an inflammatory response by the fetus. Clinical diagnosis is often difficult because the clinical signs and the pathological lesions are not specific for C. abortus infection. Laboratory diagnosis is necessary to reach a definitive diagnosis (Marsilio et al., 2005).
A Unique Life-Cycle
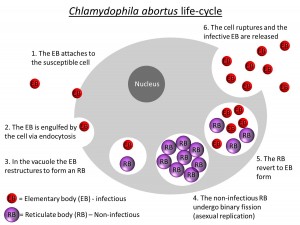
The causative bacterium, Chlamydophila abortus, has a unique life cycle involving an extracellular infective phase (elementary body (EB)) and an obligatory intracellular replicative phase (reticulate body (RB)), which is not infectious. The chlamydial infection cycle is initiated when the EB adheres to the outer membrane of a susceptible cell and is drawn into the cytoplasm. The EB occupies a vacuole in the cytoplasm, to which cellular lysosomes are not attracted, and so the normal defensive reaction of the host cell does not occur. Within the vacuole, the EB alters morphologically to RB, which is adapted for metabolic parasitism of the host cell and for division by binary fission (division in half). Following numerous divisions, the process of differentiation is reversed and the progeny RBs revert to EB forms. They are released by the cells rupturing and the infection cycle renews (Aitken, 1991).
Regardless of when infection occurs, Chlamydophilia abortus initiates the disease in pregnant ewes after 90 days into the pregnancy. Initially infection occurs in the centre of the placental cotyledons and then spreads outwards on the surface of the membranes connecting the cotyledons. The resultant damage, swelling and inflammation cause the characteristic red (sometimes also yellowish) thickened appearance of the placental membranes seen at the time of abortion. The level of the hormones progesterone, estrogens and prostaglandin controlling the onset of lambing are affected by the infection, and it is thought that this imbalance, resulting from placental damage, is the underlying cause of abortion.
The birth of a premature stillborn lamb one or two weeks before the actual lambing date is often the first sign, although there may be vulval discharge and behavioral changes up to 48 hours before. The stillborn lamb may look normal or be a bit pot-bellied in appearance due to the collection of body fluid under its skin. A chlamydial infection can, however, also produce full term stillborn or weakly lambs. It is not uncommon for an infected ewe to produce one dead lamb and one or more live, weakly or healthy lambs. Some ewes may produce healthy female (ewe-) lambs, which may carry the infection and abort in their first pregnancy.
How is C. abortus spread?
Aborting ewes contaminate the environment with the bacteria present in the vaginal discharge, which can be picked up orally by susceptible ewes, causing further spread of infection, and these will lie dormant until a subsequent pregnancy when they may cause abortion. However, infection and abortion within a single pregnancy can also occur, especially in flocks with an extended lambing period (Blewett et al., 1982). Ewes that have aborted should be culled, as they are likely to be carriers.
Control and Prevention of Enzootic Abortion
Find out the status of your flock
The status of enzootic abortion of a flock can be established by carrying out a blood test on a proportion of the sheep. If a flock is clear of infection then it is important to keep the flock clean and buy in replacements from a safe source.
What to do in an ‘abortion storm’?
In case of an outbreak, the primary aim is to prevent spread of infection. Ewes that abort or deliver a dead or weakly lamb should be clearly marked for later identification and immediately isolated from the other ewes until their uterine discharges have dried up. Aborted fetuses, dead lambs, placentas and any contaminated bedding must be removed and destroyed. Lambing pens in which abortion has occurred should be cleaned and disinfected and, if possible, not reused. If only a few ewes are involved, it may be wise to cull them. These ewes will be able to breed successfully in subsequent years but may remain carriers (Buxton, 1998).
Vaccinating against enzootic abortion
Three vaccines currently on the market include Enzovax (Intervet), Cevac Chlamydia (Ceva Animal Health) and Mydiavac (C-Vet VP). Enzovax and Cevac are live vaccines and both have a meat withdrawal period of 7 days and neither should be used in pregnant animals or less than 4 weeks before mating, and in any animals being treated with antibiotics, particularly tetracyclines. Full details of each vaccine can be located on the Noah Compendium. (for Noah Compendium click here ).
Treating Enzootic Abortion
If active chlamydial infection (e.g., evidence of abortion confirmed to be caused by C. abortus) is thought to be present in a flock of pregnant ewes, then the entire flock can be treated with long-acting oxytetracycline, which will reduce the severity of infection. For the best effect, the treatment should take place between 95 and 105 days of gestation. A further injection two weeks later will further reduce losses. However, some ewes will still abort and many may excrete infection at lambing time. Alone and in conjunction with antibiotic treatment, the use of inactivated vaccine should be considered as an emergency treatment in the face of an outbreak.
The course of action taken will have to be carried out under the supervision of a veterinary surgeon.
On farms where vaccination is recommended (e.g., where a definitive diagnosis was obtained), ideally all ewes should be vaccinated in the first year and subsequently all breeding replacements vaccinated once at any time up to four weeks before tupping with a live vaccine.
Good Practice Based on Current Knowledge
- If chlamydial infection is already in the flock, a strategy should be developed with the veterinary surgeon and included in the overall health plan to eradicate the disease using vaccination
- Ewes which have aborted should be culled, as they may be carriers
- Aborted fetuses, dead lambs, placentas and any contaminated bedding must be removed and destroyed. Lambing pens in which abortion has occurred should be cleaned and disinfected and, if possible, not reused
- In a case of the disease being present in a small flock, it may be worth replacing the entire flock
Aitken, I. D. (1991) Enzootic (Chlamydial) Abortion. In: Diseases of Sheep. 2nd edition. Ed.W. B. Martin and I. D. Aitken. pp. 43-49. Blackwell Scientific Publications, Oxford.
Blewett, D. A. Gisemba, F. Miller, J. K. Johnson, F. W. A. and Clarkson M. J. (1982) Ovine enzootic abortion: the acquisition of infection and consequent abortion within a single lambing season. Veterinary Record 111, 499-501.
Buxton D, Anderson IE, Longbottom D, Livingstone M, Wattegedera S, Entrican G (2002) Ovine chlamydial abortion: Characterization of the inflammatory immune response in placental tissues. Journal of Comparative Pathology 127 (2-3): 133-141
Chalmers, W. S. K. Simpson, J. Lee, S. J. Baxendale, W. (1997) Use of a live chlamydial vaccine to prevent ovine enzootic abortion. Veterinary Record 141: 3, 63-67.
Chalmers, G.A., Klavano, G.G., Nagge, W.T., and Christian, R.G. (1976). Ovine chlamydial abortion in Alberta. Can. Vet. J. Rev. Veterinaire Can. 17, 76–81.
Dubey, J.P., and Kirkbride, C.A. (1990). Toxoplasmosis and other causes of abortions in sheep from north central United States. J. Am. Vet. Med. Assoc. 196, 287–290.
Entrican G, Wheelhouse NM (2006) Immunity in the female sheep reproductive tract. Veterinary Research 37 (3): 295-309
Leonard, C. Caldow, G. L. Gunn, G. J. (1993) An estimate of the prevalence of enzootic abortion of ewes in Scotland. Veterinary Record 133: 8, 180-183.
Longbottom, D., Entrican, G., Wheelhouse, N., Brough, H., and Milne, C. (2013). Evaluation of the impact and control of enzootic abortion of ewes. Vet. J. 195, 257–259.
Milne, C. Dalton, G. E. (1988) The economics of enzootic abortion prevention and control. Farm Management 6: 12, 529-533.
Marsilio F, Di Martino B, Di Francesco CE, Meridiani I (2005) Diagnosis of ovine chlamydial abortions by PCR-RFLP performed on vaginal swabs. Veterinary Research Communications 29: 99-106 Suppl. 1
Nietfeld, J.C. (2001). Chlamydial infections in small ruminants. Vet. Clin. North Am. Food Anim. Pract. 17, 301–314, vi.


 British English
British English

Comments are closed.