Cattle Diseases
Infectious Bovine Rhinotracheitis
Also known as: IBR, Bovine Herpes virus 1
The virus is shed in respiratory secretions and in the semen of infected bulls. Once infected by BHV-1, an animal mounts an immune response, but the virus is not completely eliminated and remains as a latent infection. When a latently infected animal is subjected to stress, the virus is shed, although there are usually no clinical signs of disease. Latent carrier animals are important in IBR epidemiology as a source of infection.
The exposure of cattle to BVH-1 has been known to be widespread in the UK since the 1960s (Dawson and Darbyshire, 1964), but the disease was generally considered unimportant, as clinical signs were mild or rare. In the late 1970s, a more virulent form of the disease was reported (Wiseman et al., 1978), and it is believed that a more virulent strain of the virus entered the country (Edwards et al., 1990). In 1992, 34% of farms in the UK had one or more calves with antibodies against BHV-1 (Hogg, 1992). In North America the disease is widespread as well, in two Canadian provinces the seroprevalence in healthy animals was 37.8 %, with 59.5 % of the premises having positive animals (Durham and Hassard 1990).
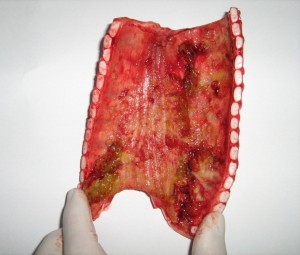
The inside of windpipe of a lactating heifer that died of IBR. Photo by P. Plate, Endell Veterinary Group
Herds with endemic IBR suffer mainly from low-grade problems associated with calf pneumonia, decreased fertility and occasional abortions. More serious problems such as milk drop, respiratory disease, abortions and an increase in calf pneumonia are seen on farms that have not been exposed to IBR before, with extremely severe and dramatic respiratory signs in some cases (Cutler and Harwood, 2000). However, sub-clinical infection and seroconversion in a naïve herd was also reported (Pritchard et al., 2003). Depending on the strain, this may or may not involve obvious clinical signs. If these herds are stressed, severe respiratory outbreaks and deaths can occur, with characteristic fibrinous lining of the trachea. On an individual cow level within infected herds, Statham et al., (2015), found that seropositive cows gave on average 2.6 litres per day less milk over a two year study period. In calves, concurrent respiratory and neurological signs have been reported consequences of BHV-1 infection, where the maternal antibody protection was suspected to be poor (Penny et al., 2002).
Control and Prevention of Infectious Bovine Rhinotracheitis
The prevention and control of IBR is based either on preventing the virus from entering the herd or on vaccination. Many European countries have eradicated or are in the process of eradicating IBR.
Preventing the Disease From Entering the Herd
Animals carrying latent BHV-1 infections can shed the virus at any time throughout their lives, particularly at times of stress, such as other disease, calving, transport etc. Entry of such a BHV-1 carrier to the herd, or direct contact with infected animals are usually the sources of infection in a herd that has not been exposed to BHV-1 before (Van Winden et al., 2005). Buying in bulls in particular is a risk as they carry a greater probability of being seropositive for IBR, possibly because bulls are often shown or mix with different herds during their lifetime (Martinez-Ibeas et al., 2015).
Screening, and Eradication
It has also been shown that in closed herds the virus circulates from infected to non-infected animals, and disease eradication has been successfully carried out in beef herds by segregating the seropositive and seronegative animals (Corkish, 1988; Ackerman et al., 1990).
If the disease is present, all animals over 12 months of age are tested with seropositive animals removed. Two clear tests (1-12 months apart) are required for formal accreditation of freedom from disease under the UK Cattle Health Certification Standards (CHeCS). Annual monitoring is undertaken to ensure continued freedom of disease. Once free of disease, high standards of biosecurity are required to prevent IBR entry. If there is a high prevalence of seropositive animals at initial screening, vaccination using a marker vaccine can be employed to aid the eradication process (see vaccination below).
At a lower cost and with less stringent biosecurity regulations, a farm can also be a part of a screening and eradication programme for IBR with the same organisation.
Vaccination of Infectious Bovine Rhinotracheitis
Whilst vaccination is an effective way of controlling the disease, it does not stop infected animals from shedding the virus and is not a guarantee against introduction of the disease into a herd.
Therefore attempts to prevent the disease from entering the herd should be based on good biosecurity.
There are live and dead vaccines available, and a specific vaccination protocol for prevention, control or to aid in eradication of the disease should be discussed with your vet as part of ongoing health planning. It is possible to administer some of the vaccines intranasally and this route of administration is often used in the face of an outbreak, acting quickly and helping to reduce the number and severity of new cases.
Screening of animals for IBR exposure cannot differentiate between antibodies produced by conventional vaccines and real infection with BHV-1 and should NOT be used as part of an eradication programme. However, the IBR marker vaccines do allow differentiation between natural infection and vaccination and can be used in an eradication programme (Gehrmann et al., 2003; Simon, 2004). Marker vaccines have a protein, Glycoprotein E (gE), deleted from the vaccine virus, but this is present in the virus of natural infection and conventional vaccines. Therefore, by examining blood for the presence of antibody to gE the positive animals are only those that have been exposed to natural infection or conventional vaccines. It is important to remember that some multivalent pneumonia vaccines may include a conventional IBR virus component and it is very important to avoid inadvertently administering a conventional IBR virus vaccine in this way if eradication is desired. It is also worth noting that even the use of a marker vaccine may disqualify an animal for export into some EU countries.
Treating Infectious Bovine Rhinotracheitis
There is no specific treatment for IBR. During an outbreak, the use of broad-spectrum, long-acting antibiotics can prevent secondary bacterial pneumonia. Additionally, used of a non-steroidal anti-inflammatory (NSAID) may help relieve respiratory symptoms and pyrexia.
Vaccinating cattle during an outbreak may reduce new cases but is not helpful in animals that have clinical symptoms (Van Donkersgoed and Babiuk, 1991). An intranasal vaccine is likely to prevent new cases within 24 hours.
Infectious Bovine Rhinotracheitis and Welfare
Affected animals should be isolated and treated to protect them from secondary bacterial infections, preferably with broad-spectrum antibiotics and NSAIDS.
Good Practice Based on Current Knowledge
As part of the herd’s Health Plan, find out and monitor what the herd’s IBR status is (blood sampling in beef herds and bulk milk sampling and blood sampling in dairy herds). To prevent IBR from entering the herd:
- Implement a closed herd policy
- If necessary, purchase replacement stock from herds certified free of IBR (i.e., accredited herds)
- Quarantine all added animals for 4 weeks and test them for IBR antibodies before inclusion into the main herd
- Avoid direct or indirect contact with cattle from potentially infected farms (shows, markets, contact over fences, rented grazing, hired bulls, etc.).
- Talk to your neighbours
- Do not allow the introduction of disease via AI technicians, vets, hoof trimmers, visitors etc. (e.g., dedicated footwear for people who enter cattle housing, disinfection of protective clothing and equipment) and limit access to essential visitors only
- Isolate cattle from truck delivery and pick-up points


 American English
American English



Comments are closed.