Cattle Diseases
Bovine Tuberculosis
Also known as: TB, bTB
Control of bTB is important because of its potential zoonotic and also because of the severe economic effects of slaughter and movement restrictions required to control the disease. M. bovis is spread in a number of ways by infectious animals – in their breath, milk, discharging lesions, saliva, urine or feces. Cattle can become infectious long before they exhibit any obvious clinical signs or lesions typical of bTB, even with the most careful vet inspection (de la Rua-Domenech, 2006). However, they can spread disease around 87 days after infection occurs (Neill et al., 1991). Entry is usually by inhalation (especially if housed) or ingestion (when wildlife is the source of infection). Once in a herd, infection probably spreads from cow to cow by inhalation (Costello et al., 1998), and transmission from cows to calves may occur via the milk or colostrum (Evangelista and De Anda, 1996).
Clinical signs of bTB?
Control and Prevention
Pre- and post-movement testing
bTB Spread from Cattle
bTB Spread from Wildlife
Treatment Options
Good Practice
While once TB was the most prevalent infectious disease of cattle and swine in the United States, it has now been nearly eradicated from livestock in the US through a cooperative state-federal program. In 2009, bTB herd prevalence in the U.S. had decreases to an estimated 1 in 100,000, and in cattle had decreased to an estimated 1 in 1,000,000 (0.00001%) (Humphrey et al., 2014). However, vigilance is still necessary. In the UK, the disease incidence is increasing, particularly in cattle dense areas such as in the south west. The table below shows the number of herds and cattle routinely tested in the UK between 2003 and 2012, and the number of cattle slaughtered because they were reactors, or in direct contact with reactors. The UK government publish a series of national and regional statististics regarding the incidence of bTB in Great Britain. To access them click here.
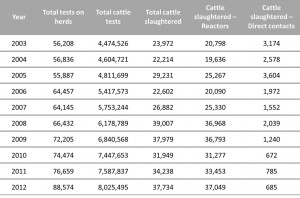
This table shows the number of herds tested, cattle tested, cattle slaughtered and of those how many were reactors or direct contacts in the UK in the period between 2003 and 2012. This table has been adapted from TB in Cattle in Britain DEFRA
The bacterium M. bovis is able to remain viable for long periods in moist and warm soil and it can survive in a wide range of acids and alkalis. However, it is killed by UV radiation from the sun. In cattle faeces it can survive 1 – 8 weeks (Andrews, 1992) and may survive in drinking water for up to 18 days (Cobner, 2003). M. bovis has been found in several wild mammal species (Cobner, 2003), although it is thought that with the exception of badgers and possibly deer, the prevalence of infectious individuals amongst most common farmland wildlife species is extremely low and unlikely to maintain disease as an important reservoir for cattle (Mathews et al., 2006).
High rates of infection have been found in badgers and the consensus of scientific opinion is that badgers are a significant source of TB in cattle (Clifton-Hadley et al., 1995; Cobner, 2003; Denny and Wilesmith, 1999; Martin et al., 1997; Ó Máirtı́n et al., 1998). Animals are probably more likely to be infected by M. bovis when they are poorly nourished or under stress. Growing heifers and younger cows are most at risk (Griffin et al., 1996). There is evidence that more intensive dairy farms have a higher risk of infection (Griffin et al., 1993). The strongest factors associated with increased bTB breakdown risk are:
- Movement of cattle onto farms from markets or sales
- Operating a farm over multiple premises
- The use of close contact housing (Johnston et al., 2005).
M. bovis can infect people (Chalmers et al., 1996; Hardie and Watson, 1992) and was in the past a major cause of death in humans in the United Kingdom mainly through the consumption of raw, unpasteurised milk. The total incidence of human TB in the UK remains high compared to other Western European countries, with 8,751 cases reported in 2012 (Ref: UK TB Surveillance data). Of these 5,200 cases (59.4%) were culture confirmed and of these 39 cases were identified as M. bovis, the agent of bovine tuberculosis (ref: Public Health England ).
Clinical signs of Bovine Tuberculosis in Cattle
Tuberculosis is rarely diagnosed clinically. Various body systems can be affected, but signs are usually confined to the respiratory tract. A soft, chronic cough occurs once or twice at a time. In more advanced cases, there is a marked increase in the depth and rate of respiration as well as dyspnoea. Areas of dullness can be heard in the chest on auscultation or percussion. Some cases may squeak, whistle or have a snoring respiration (Andrews, 1992; Cassidy et al., 1999). Lymph node enlargement may present as localized swellings, or may cause dyspnoea or dysphagia (Cobner, 2003).
Control and Prevention of Bovine Tuberculosis
Systematic and routine testing aided by compulsory slaughter of reactors, movement restrictions for affected herds and slaughterhouse surveillance. bTB is a notifiable disease and any suspect cases should be notified to the authorities.
There are two types of bTB tests used internationally, the skin test and the IFN-gamma test.
The bTB skin test involves a small volume of bovine tuberculin (M. bovis protein derivative) being injected into the skin and then the immune reaction is measured 72 hours later. This can either be a single intradermal test (SIT) or a single intradermal comparative cervical test (SICCT). The SICCT is a separate but simultaneous injection into the skin on the neck of the cow of both bovine and avian tuberculin next to each other. Some cows may be exposed to other mycobacterium species (that are not M. bovis), so a comparative test can discriminate between these cows and should help to minimize false positives. With both skin tests, the vet must revisit the farm 3 days later to measure any localized swelling caused by an immune response. The presence and size of this swelling will determine if the cow is a reactor, inconclusive reactor (IR) or TB free.
The type of skin test (SIT or SICCT) selected depends on the prevalence of TB, and on the prevalence of other environmental mycobacteria. In the USA, Canada and New Zealand the caudal fold SIT is the primary test for routine TB screening. In the UK and Ireland, the SICCT test is used (de la Rua-Domenech et al., 2006).
The IFN-gamma test is often used as an ancillary (extra) test to the skin test and involves a blood sample being taken from the animal, and this is immediately transported to the lab where it is incubated in the presence of M. bovis antigens. The level of IFN –gamma detected in these samples determines if the animal has been previously exposed to M. bovis antigens. The advantages of this test over the skin test are only one visit is needed to the farm, it can detect infection 1-5 weeks after infection unlike 3-6 weeks for the skin tests, and repeat tests can be carried out immediately after, instead of a 60 day break for the skin tests. The disadvantages of the IFN-gamma test include a lower specificity (produces more false positives) and the challenge of keeping the blood samples between 10 and 26°C until testing. Also a small number of cattle that react to the skin test are undetected in the IFN-gamma test (Pollock et al., 2005).
In the UK, animals which react to the skin test (Reactors) are compulsorily slaughtered, and restrictions are put in place (a TB2 Restriction Notice is issued to the farm) so that animals may not be moved on or off the affected farm (except direct to slaughter under license) until further tests show that the herd is clear. After a reactor is confirmed by post mortem and/or culture a herd must have two clear tests 60 days apart before restrictions are lifted. Some cows that react slightly but not enough to be a Reactor are deemed ‘Inconclusive Reactors’ (IR). All Reactors and IRs must be isolated from the rest of the herd immediately. Milk from reactors is unfit for human consumption.
What happens next depends on whether or not confirmed reactors have been identified in the herd, and if the herd has had a TB breakdown in the past 3 years. If not and no other reactors have been identified, the IRs will be put under restriction and will be tested again in 60 days. If the second test is clear these cows can rejoin the herd. This process seriously disrupts farm business and can be very expensive. That is why it is important to try and prevent the disease from spreading. bTB can be spread from cattle to cattle, or from wildlife to cattle.
Pre-movement and post-movement testing in the UK
Pre-movement and post movement testing are in pace in the UK to reduce the risk of spreading TB between herds and to areas free from disease. Below is a summary of the pre-moment test currently in place in England:
- In England and if you have a herd that is under the yearly bTB testing regime, you probably to have a pre-movement test within 60 days prior to moving your cattle. There are exemptions, such as cattle under 42 days old, and those that are going to slaughter but full details can be found on Defra’s website.
- The routine annual herd test can be used as the pre-movement test as long as it is within 60 days before the movement.
- The skin test is the only test that is licenced for the pre-movement test and it must be carried out by an official vet registered with the APHA (Animal and Plant Health Agency) cattle testing panel.
Cattle to Cattle Spread of Bovine Tuberculosis
 There is conflicting evidence about risk factors. More intensively farmed cattle are more at risk from the disease according to Griffin et al., 1993. Lower stocking rates may be beneficial in reducing the risk of TB infection. However Reilly and Courtenay (2007) identify higher stocking densities as well as mixed enterprise as a protective risk factor. There is agreement that buying in cattle is a risk factor. The prevention of contact between cattle on neighboring farms is very important. Sound fencing to stop nose to nose contact and barriers in gateways to stop contact with passing cattle are important. Contract reared cattle should be kept away from the herd.
There is conflicting evidence about risk factors. More intensively farmed cattle are more at risk from the disease according to Griffin et al., 1993. Lower stocking rates may be beneficial in reducing the risk of TB infection. However Reilly and Courtenay (2007) identify higher stocking densities as well as mixed enterprise as a protective risk factor. There is agreement that buying in cattle is a risk factor. The prevention of contact between cattle on neighboring farms is very important. Sound fencing to stop nose to nose contact and barriers in gateways to stop contact with passing cattle are important. Contract reared cattle should be kept away from the herd.
A closed herd system and breeding own replacements should help to keep the disease away from the farm. Bought-in animals should be quarantined away from the main herd as part of the herd’s normal Biosecurity control measures detailed in the Herd Health Plan. Legislation in England, Scotland and Wales requires that all cattle over 42 days old moving out of a yearly tested herd must have tested negative to a TB test within 60 days prior to movement unless the herd or movement meets any of the exemptions (DEFRA Pre-Movement Testing). In Scotland, the Scottish Executive and Rural Affairs Department introduced compulsory post-movement testing in addition to reduce the chance of importing previously undetected bTB from areas that have a relatively greater incidence of the disease. Routine TB surveillance tests qualify as pre-movement tests, if animals are moved within 60 days of that test.
If possible, avoid common grazing. If common grazing is inevitable then skin tests should be synchronized on all farms using the common grazing.
Bovine Tuberculosis Spread from Wildlife
Bovine tuberculosis is endemic in white tailed deer and this has tremendous consequences for the livestock industry (VeerCauteren et al., 2008). The free-ranging white tailed deer in Michigan, USA has been under surveillance for over a decade since it was confirmed to be a maintenance host, also known as the wildlife reservoir. The apparent prevalence in white-tailed deer has reduced by >60% primarily through the cull of deer and restrictions on public feeding and baiting of deer. However, public resentment of the control measures has grown (O’Brien et al., 2011). As well as the white-tailed deer, bovine TB outbreaks in captive cervids have been increasingly found in the US in recent years, including multiple herds of elk in Indiana, a mixed herd of red deer and fallow deer in New York, and a farm of elk and fallow deer in Nebraska (Lyashchenko et al., 2013).

Areas around badger sets should be fenced to keep cattle out while allowing badgers free access. Contact with high-risk animals should be minimized at all times.
In the UK M. bovis has been isolated from a large range of native mammals including deer, moles, rats, foxes, ferrets, minks and farm cats. However, there is compelling evidence that the European Badger is the major wildlife reservoir and one of the main reasons for the TB breakdown in England and Wales. M. bovis is spread through saliva, sputum, urine, and feces of infected badgers. Direct observations and video surveillance has proven that badgers enter cattle sheds and feeds store where infected animals are able to shed M. bovis enhancing the likelihood of disease transfer (Delahay et al., 2001).
In order to reduce TB, contact between badgers and your livestock needs to be minimized. In the case of badgers it is particularly useful to know where the setts are and which areas they utilize as latrines. Areas around badger sets should be fenced off to keep cattle out while allowing badgers free access. Badger latrines should also be fenced off for cattle (Hutchings and Harris, 1999). Active latrines are avoided by most cattle until the sward length in the rest of the field has reduced, after which there is an increasing likelihood that active badger latrines are grazed, especially by lower ranking cows (Hutchings and Harris, 1997). Overgrazing fields used by badgers should therefore be avoided. When cutting for hay or silage, grass from badger latrines and field margins should be also be avoided. Additionally badgers and other wildlife should be kept out of farm buildings, and doors should be secure and kept closed at night. It is also advisable to raise feed and water troughs so that their lips are at least 80 cm off the ground.
A review of measures to reduce badger to cattle transmission can be found at Ward et al., 2010.
Treating Bovine Tuberculosis
All animals affected by tuberculosis are compulsorily slaughtered, and restrictions are placed so that animals may not be moved on or off the affected farm (except direct to slaughter under license) until further tests show that the herd is clear.
Good Practice based on Current Knowledge
Reducing Cattle to Cattle Spread of Bovine Tuberculosis
- Establish a closed herd system and breed own replacement. Every purchase is a risk
- Prevent contact between your cattle and those on neighboring farms; use sound fencing to stop nose to nose contact; place barriers in gateways to stop contact with passing cattle
- Keep contract reared cattle away from your herd
- Isolate any bought-in animals
- Check when bought-in cattle were last TB tested. If they need testing before your routine herd test, consider a private TB test, or consider whether a compulsory pre-movement test is required
- If you share livestock vehicles, ensure they are disinfected
- If possible, avoid common grazing, however if it is inevitable, then routine skin tests should be synchronized
- Tell neighbors if you have a TB breakdown to enable them to take precautions
- If a breakdown occurs, do not use pooled colostrum to feed calves as this may spread disease
Minimizing Spread of bTB from Wildlife
- Keep wildlife out of farm buildings, especially feed stores. Keep the doors secure and closed at night
- Fence off areas around badger setts to keep cattle out while allowing badgers free access
- Fence off clusters of badger latrines
- Overgrazing fields used by badgers should be avoided
- Avoid grass cutting for hay or silage from badger latrines and field margins
- Raise feed and water troughs so that their lips are at least 80 cm off the ground, and keep them clean
- Silage clamps should be well covered and the face protected if not used
- Molasses blocks should be placed where they are difficult to be accessed by badgers


 British English
British English

Comments are closed.