Cattle Diseases
Liver Fluke in Cattle
Also known as: Bovine Fasciolosis, Fasciola hepatica
Bovine fasciolosis is an economically important disease of cattle, which can be associated with particular regions throughout the world directly linked to the habitat of an aquatic mud snail. In Europe the snail species naturally infected with Fasciola hepatica (Liver fluke) is Galba trunculata, and in the USA multiple fresh water snail species belonging to the genus Lymnaea have been reported to harbor infection (Dunkel et al., 1996). In the UK bovine fasciolosis was historically more prominent in the wetter western half, although in recent years an increase in the number of cases of fasciolosis have been reported across the UK (Pritchard et al., 2005). This may be a result of a warmer, wetter climate, especially in the summer and movement of infected sheep (Daniel, 2004). One study suggests that 50-80% of dairy herds in England and Wales have lactating dairy cows that have been exposed to Fasciola hepatica infection (Salimi-Bejestani et al., 2005). Calves and yearlings are most commonly affected, but any age of animal may be susceptible. Climatic and hydrological factors play an important part in the epidemiology of the disease.
Grazing Management
Snail Habitat Management
Monitoring for Fluke Infection
Treatment Options
Good Practice
Liver Fluke Life Cycle
- The adult fluke resides in the bile ducts in the liver of the definitive host
- The eggs they shed are passed down the bile ducts and into the intestine to be excreted in the feces. One fluke can pass between 5000 and 20,000 eggs per day
- When environmental conditions are suitable (wet and >10⁰C), the fluke eggs hatch into small infective larvae (miracidia)
- The miracidium actively seek a specific mud snail intermediate host (Galba trunculata in Europe)
- In the digestive tract of the snail, the miracidia develop into cercaria
- From one miracidium, six hundred or more cercaria are produced, emerging from the snails after about 6 weeks, depending on the climate
- The cercariae swim to attach themselves to herbage where they lose their tails and secrete a tough cyst wall to become metacercariae
- At 12-14 °C, up to 100% of metacercariae can survive for six months, though only 5% survive for 10 months. For prolonged survival, the relative humidity needs to be above 70%
- Once ingested by cattle, the immature fluke burrow through the gut wall and pass to the liver. They are voracious feeders and migrate through the liver parenchyma to reach the bile duct, where they mature. Egg laying commences some 10-12 weeks after the initial infection, which is of diagnostic importance.
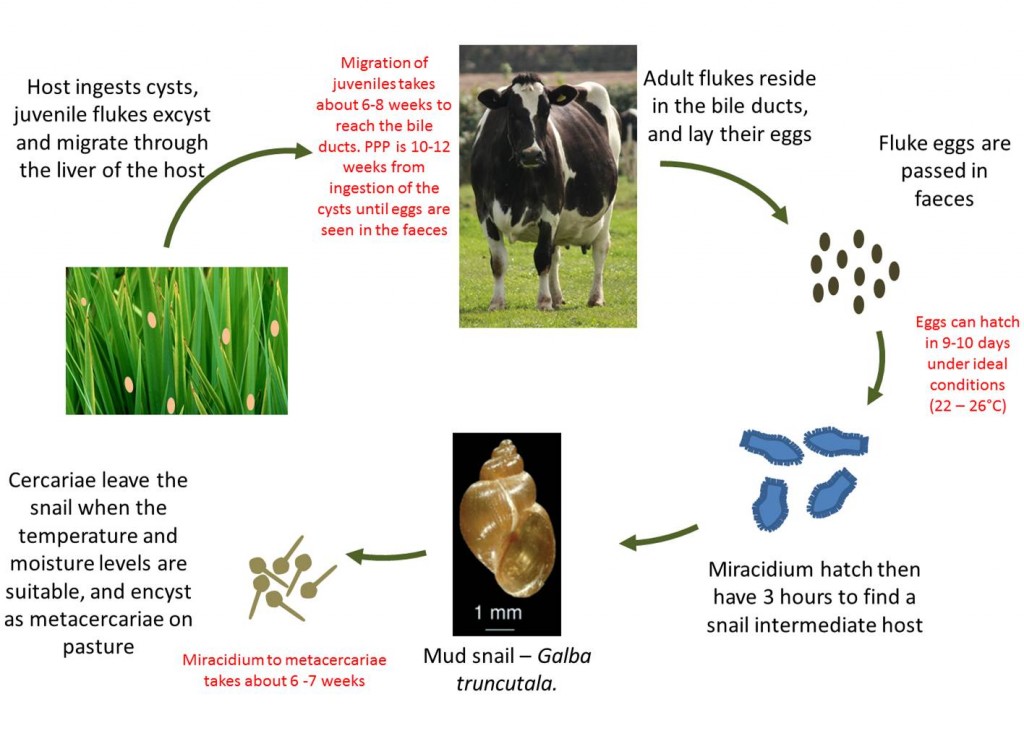
The length of the complete life cycle of Fasciola hepatica (Liver fluke) is very long and varies depending on the season as it requires a definitive host (cattle or sheep) and an intermediate host (mud snail), although the minimum period for the whole life cycle is 5 – 6 months. The time from ingesting infective metacercariae cysts on the pasture to adult flukes laying eggs in the bile ducts is a bout 10 – 12 weeks. Fluke thrives in wet summers and is wholly dependent on the presence of the mud snail (Galba trunculata in Europe)
The mud snail requires water and warm conditions for reproduction. The ‘summer’ infection of snails results from the hatching of overwintering eggs passed from the cattle (or eggs passed) in the spring. The metacercariae then appear on pasture from August to October.
A smaller ‘winter’ infection of snails is due to the infection of snails from eggs shed by the cattle in the autumn. It is thought that larval development in the snails ceases during the winter months and commences again in spring resulting in pasture contamination with metacercariae in May and June. This is significant as the infected snail hibernates carrying infection through the winter.

Liver fluke – Fibrotic bovine liver The longevity of fluke in cattle is about 1-2 years. When the bovine liver is infected, it provokes an immune response leading to a fibrotic reaction which prevent the fluke from migrating and feeding (Image from Andrew Forbes, University of Glasgow).
Acute fasciolosis is uncommon in cattle, unlike in sheep where it is a highly pathogenic . Cattle develop a greater fibrotic reaction in the liver compared to sheep, therefore parasite survival is reduced. There is limited development of immunity to fluke, which does not prevent infection. Additionally, the immunity may wane at housing, therefore all age groups of cattle are potentially at risk of fluke infection (Daniel, 2004).
The clinical signs of chronic fasciolosis are variable and depend upon the number of metacercariae ingested, but often include:
- Weight loss
- Anemia
- Bottle jaw
- Diarrhea
- Constipation (Torgerson, 1999).
Fluke infection may predispose to other conditions due to impaired liver function and can reduce milk yield and fertility (Schweizer et al., 2005). In one dairy herd, subclinical ketosis was attributed to fluke infection of the cows (Mason, 2004).
Definitive diagnosis is based on post mortem examination. Fluke egg counts in feces can be useful indicators of infection, although false negatives do occur as the infection may not be spread evenly throughout a herd (Daniel, 2004). A coproantigen test on feces is also available which will detect fluke before a standard fecel egg count. Blood and milk serological tests are also available and can be very useful at certain times of the year, although they only indicates previous exposure, not current infection.
Control and Prevention of Liver Fluke in Cattle
Fluke prevention and control should be part of an integrated approach (in conjunction with the farm vet) to parasite control in the Herd Health Plan on any farm. Control should be farm specific and farmers must consider all livestock together as liver fluke infects cattle, sheep and other grazing animals (including rabbits and deer). Fluke control measures can be divided into 3 sections:
1. Grazing management
2. Snail habitat management
- Fecal egg counts
- Coproantigen tests
- Blood and bulk milk serology
- Abattoir condemnations
- NADIS parasite forecast
Grazing management – avoid grazing high risk pastures
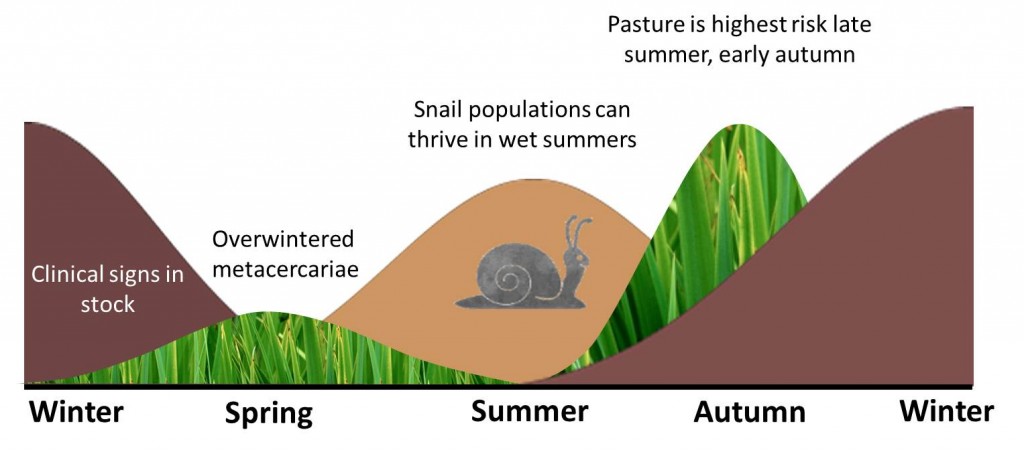
Summer infection of snails primarily leads to winter disease in cattle. Under optimal conditions Galba trunculata snails can lay 3000 eggs per year. These eggs hatch and mature within 2 weeks, and snails can be sexually mature within 3 weeks. One snail can produce 25,000 descendants within 12 weeks. They hibernate throughout the winter then re-emerge in spring. When the temperature is 10°C, the cercariae are released from the snail, and encyst on pasture as infective metacercariae. Once ingested the juvenile flukes take about 6 – 8 weeks to react the bile ducts, where they mature into adult flukes and begin feeding and reproduction.
Pasture is considered to be highest risk between late summer and autumn due to the summer infection of snails and their sheer fecundity. This leads to disease being most prevalent from autumn onwards through the winter. These risks are much lower following a hot dry summer.
Winters in Northern Europe and similar temperate climates are generally too cold for liver fluke to develop. However, low levels of metacercariae can overwinter on the pasture if the climate conditions are suitable. This along with autumn infected snails hibernating, then shedding cercariae in early spring can lead to high enough levels of metacercariae on pasture to cause low levels of clinical disease in ruminants throughout the summer.
Keeping stock off areas that were wet in late summer early autumn (when the snails released the cercariae) will help reduce the incidence of disease.
Grazing management – Avoid co-grazing
Unlike most other parasitic diseases of ruminants, liver fluke is not host species-specific and grazing sheep with cattle on high risk pasture can potentially amplify the disease. There is little evidence to suggest that cattle or sheep develop significant immunity to liver fluke. As sheep shed higher numbers of liver fluke eggs than cattle, co-grazing would benefit sheep but could be detrimental to cattle.
Snail habitat management – Fence off wet areas
Unlike with parasitic gastroenteritis, liver fluke can only be found in suitable areas where the mud snail lives. The preferred habitats of Galba trunculata are shallow water, ditches, banks of slow moving streams, spring swamps and reeds. In addition well and cattle watering troughs can provide suitable habitats (Knubben-Schweizer and Torgerson, 2014).

Wet, marshy, shallow water with a low pH, and unconfined pasture makes the ideal habitat for Galba trunculata. Reeds are often seen in this type of habitat.
Identify this ‘flukey’ pasture, i.e., wet, marshy, muddy areas, often with lots of rushes growing there, and fence this area off. It will be costly in the short term but could be beneficial in the long term. However, it is clear that this is not possible on many farms as it would mean loss of significant grazing.
Snail habitat management – Drainage of wet areas
Drainage of wet areas that are suitable habitats of the mud snail can be highly effective, but again will be costly in the short term but will help with liver fluke control in the long term. However drainage may not be permitted under some environmental schemes as it could be detrimental to other wildlife habitats.
Monitoring for infection – Fecal egg counts
Regular fecal samples should be taken from the cattle to determine the liver fluke burden on the farm, however FECs are limited as they will only confirm an infection (if eggs are seen in the fecal samples) but do not give an indication of parasite burden and if eggs are not presence in the feces, it does not confirm there is not a fluke infection. Only adult fluke produce eggs, and as they reside in the bile ducts, the eggs are only passed out sporadically. This aside composite FECs from 10 or more animals can be useful to monitor incidence of liver fluke on farms. Due to the seasonality of liver fluke and the fact that they commence shedding eggs 10-12 weeks post infection, FECs are more useful at certain times of the year (e.g., winter and spring).
Monitoring for infection – Blood and bulk milk serology
While FECs are not very sensitive, but definite if possible, individual blood serology detects antibodies against liver fluke from two weeks post infection. They can be useful at certain times (e.g., before housing). Liver fluke serology is very sensitive (positive at exposure) and titres become positive from two weeks after infection. Therefore they are a good indicator whether infection occurred (mainly in the autumn) and if a treatment during the housing period is necessary. This treatment should eliminate liver fluke if the right product is chosen (e.g. triclabendazole two weeks after housing, other products later). Egg counts take 10-12 weeks to become positive (prepatent period), so late infections just before housing would not be detected by an early housing test, and the test is less sensitive, so positive animals can be missed.
Monitoring for infection – Coproantigen tests
This is a relatively new commercially available test which detects fluke digestive enzymes in host fecal samples. It is useful to diagnose individual cases but has not been fully evaluated on mob fecal samples. However, coproangtigen tests will detect fluke infection about 3 – 6 weeks before FEC.
Monitoring for infection – Abattoir reports
Abattoir reports can be used as an indication of infection on a farm, however they usually condemn the liver and report with active or historical fluke infection. Also a liver condemned for fluke does not always mean a high level of infection; it could just mean that one adult fluke was seen. Nevertheless it is an indication that fluke (and the mud snail!) is on the farm, and further investigation is required.
Monitoring for infection – NADIS parasite forecast
Fasciolosis forecasts by NADIS animal health are based on the Ollerenshaw predictive model that relates meteorological data to the probable incidence of fasciolosis in particular years. These are published on the NADIS website here.
Quarantine of stock – Biosecurity
Buying in stock brings with it the risk of disease onto a farm that was not present before. This includes the introduction of resistance parasites. Quarantine of bought-in stock should always be undertaken as a biosecurity measure and if there is any doubt over the fluke status, treatment should be considered.
Treating Liver Fluke in Cattle

Fasciola hepatica is not (definitive) host specific, it can infect both cattle and sheep, although has more severe effects in sheep. It can also infect horses, mice, rabbits and deer. There are also between 2.5 and 17 million reported cases of human fasciolosis from eating aquatic vegetation (mainly wild watercress) contaminated with infective metacercariae (Ghildiyal et al, 2014).
Flukicide treatments are the most common method of control but they do not have persistent activity so will not prevent re-infection, and triclabendazole resistance has been reported in sheep and cattle (Mitchell et al., 1998; Moll et al., 2000; Thomas et al., 2000, Gordon et al., 2012). All flukicides will effectively kill adult fluke, some (nitroxynil and closantel) will kill the late immature stages but only triclabendazole (which is used extensively in the sheep sector because of the high mortality of acute fasciolosis in sheep) is the only product effective against very early juvenile stages (2 weeks post infection onwards).
There has been increasing interest in plants with molluscicidal (snail-killing) activity, such as some Eucalyptus spp. (Hammond et al., 1994) and the latex of Euphorbiales spp. (Singh and Agarwal, 1988). However, this research is in its early stages and there is no work quantifying the effect of these plants in the field. More research is required.
Certain sciomyzid fly larvae have been shown to eat mud snails, and this has been put forward as a possible method for the control of snails. Work has been conducted in Ireland on Ilione albiseta to see if this insect could be exploited in the control of fasciolosis (Gormally, 1987, 1988a, 1988b).
Research is being undertaken to develop a vaccine against secretary proteins, which would prevent fluke survival. There are still significant problems to overcome, but there do appear to be some realistic prospects of producing a vaccine against F. hepatica (Mulcahy et al., 1999, Jarayaj et al., 2010). This will be useful to farmers who do have a continuous problem with chronic bovine fasciolosis.
Flukicides – Strategic treatments to reduce pasture contamination

Adult cattle do not build immunity to bovine fasciolosis. Due to the long milk withdrawal periods associated with many flukicides, it is recommended that if fluke has been identified as a problem on a dairy farm, that cattle are given a flukicide at drying off. In the UK recent changes in legislation only allow one product to be used in dairy cattle at drying off (Fasinex 240). For infection in lactating cows an adulticide has recently been licenced. Oxyclosanid (Zanil) and some white drenches like albendazole can be given to lactating cows if needed.
Strategic dosing schemes have been reported to help reduce fluke burdens over a period of four years, such that overall treatment frequencies and fluke burdens could be reduced (Parr and Gray, 2000).
Dairy Cattle
In dairy cattle which calve in the spring, treatment at drying off in the winter can be recommended. Young stock can be treated at the same time. Cows calving at other times of the year are more difficult to treat, as they may be lactating during the winter housing period. It may be possible to treat selectively cows with the heaviest burden of parasites (judged by fecal egg count) rather than blanket treating the whole herd. This will require close observation and regular fecal egg counts during the risk period. Only Abendazole and Oxyclosanide can be used during lactation (although they have long withdrawal periods, which makes use difficult), and these are only effective against adult fluke. In certain areas with high infestations, consideration should be given to synchronizing the calving pattern during the conversion period, so that the animals are not lactating during the period of maximum transmission in the autumn when treatment (against immature and mature fluke) can be given. Together with other management changes, this may be enough to keep liver fluke under control (Torgerson, 1999).
Beef Cattle
In beef cattle, the first treatment should be given at housing or, if the cattle are not housed, during late autumn and early winter. A second treatment in the spring will kill any fluke that have survived the housing treatment or, in the case of out-wintered cattle, is the result of reinfection. A product that only kills adult fluke will suffice for this treatment. These cattle will not be able to infect the pasture. In some areas, a third treatment in the summer may be necessary to kill any fluke which result from the winter infection of snails. Over time, the number of treatments may be reduced to a single treatment at housing, and eventually as other on-farm control measures are put into place, treatment may be dispensed with.
Flukicides – Therapeutic treatment
Therapeutic treatments are for those animals showing signs of disease, and for animals who are not showing overt signs of disease but have sub-clinical disease which is affecting production and may pre-dispose to other diseases. Following clinical cases of bovine fasciolosis, it is likely that the whole herd is suffering from the infection, although there may be large individual differences. This can be checked by fecal egg counts. Regular treatments are initially given at 12-13 week intervals to reduce the intensity of infection in the herd. The number of treatments may be reduced following the reduction in prevalence of liver fluke (Torgerson, 1999).
Fluke burdens can vary enormously on the same farm from year to year, therefore constant surveillance and monitoring should be encouraged, by means of forecasting, fecal sampling and abattoir feedback.
Good Practice Based on Current Knowledge
- Fence off snail habitats or exclude stock from fluke areas at periods of high risk of infection where possible
- Keep an eye on the fluke forecast for the year
- If necessary, work out a strategic worming regimen against liver fluke with the farm vet
- Herds with clinical cases of bovine fasciolosis require regular treatments with a flukicide that kills both mature and immature fluke until prevalence is reduced
- Fecal egg counts are advisable on a regular basis to follow disease trends
- Where possible, obtain feedback from abattoirs where cattle are killed to obtain information on liver fluke prevalence
- It may be possible to treat cows on an individual basis following fecal egg counts rather than treating the whole herd. This will require close observation and regular fecal egg counts during the risk period.


 British English
British English


Comments are closed.