Parasitic Gastroenteritis in Ruminants
Including: Cooperia onchophora, Ostertagia ostertagi, Teladorsagia circumcincta, Trichostrongylus spp., Haemonchus spp. (Barbers Pole Worm), Nematodirus battus
Gastrointestinal nematodes (GI nematodes or gut roundworms) are major contributors to reduced productivity in cattle, sheep and goats all over the world. Parasitic Gastroenteritis (PGE) is the condition caused by large numbers of gastrointestinal nematodes that reside in the gut (abomasum and intestines) of the ruminant host. The clinical signs of PGE vary depending on nematode species and abundance. PGE is primarily a disease of lambs and first season grazing cattle. The most profound effect of parasitism in both sheep and cattle is the sub-clinical production losses (i.e., not visually obvious), the true extremity of which farmers are unlikely to be aware (Waller and Thamsborg, 2004).
Understanding Parasite Biology
The main species of GI nematode that are of veterinary importance in temperate climates are:
- Cooperia onchophora (Cattle)
- Ostertagia ostertagi (Cattle)
- Teladorsagia circumcincta (Sheep)
- Trichostrongylus spp. (Sheep and cattle)
- Haemonchus spp.(Mainly sheep but sometimes cattle)
- Nematodirus battus (Mainly sheep but sometimes cattle)
Some species of gut parasite have their own disease page on Farm Health Online as their epidemiology and clinical signs do vary. However, nematode infections are almost always a mixture of species so this page provides a broad overview of GI nematodes in cattle and sheep.
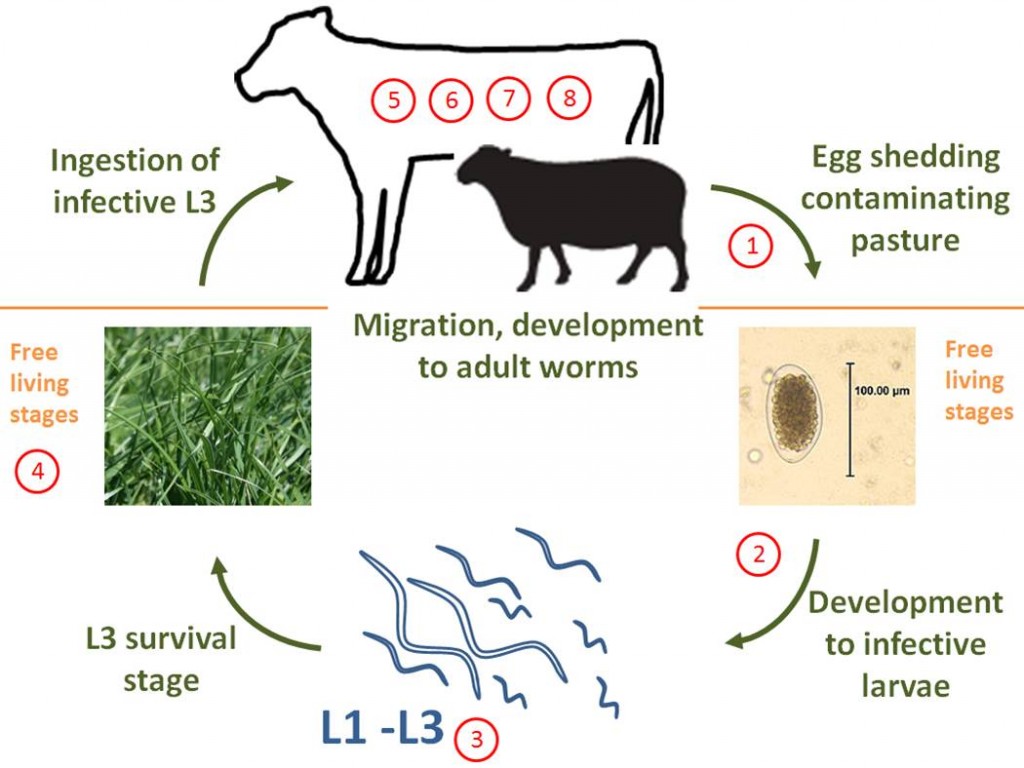
The Life Cycle of GI Nematodes
Most GI nematodes have a simple direct lifecycle, although there are species-specific variability in development rates and predilection site. The lifecycle has two distinct phases; 1) within the host, and 2) the free-living stage where the parasite is developing in the environment. The basic parasitic gut worm lifecycle follows the steps below:
- Unembryonated eggs are passed from the host in feces*
- The eggs embryonate and develop into first stage larva (L1)
- The L1 undergo two more molts (L2 and L3), and hatch out of the egg shell and migrate out of the fecal pat onto the pasture as L3
- The L3 are infective at this stage and need to survive on the pasture until ingested by the ruminant host
- Once consumed the L3 migrate to the gut** and borrow into the gut lining
- Here they undergo two more molts (as L4 and L5) before emerging*** and maturing into adult worms
- The male and female worms reproduce
- The female worms lay eggs which are passed out in the feces
*Fecal samples can be collected and the number of eggs can be counted – this is called a Fecal Egg Count. These can be used to monitor worm burden and make treatment decisions. However FECs do have their limitations especially in cattle.
**Exact predilection site depends on worm species (e.g., abomasum or small intestine)
***This is where the pathogenesis occurs
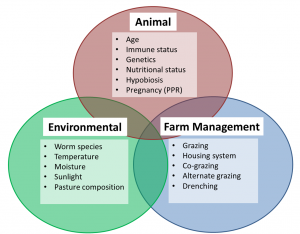
Factors affecting PGE epidemiology
The development of parasite within the host, or through the free-living stage is dependent on many different risk factors and these can be divided into 3 groups:
Animal Factors
Age-related Immunity and PGE
Unlike sheep and cattle goats do not develop age-related immunity.
Young animals in their first grazing season are most susceptible to PGE and then as they reach about 12 – 18 months they often develop strong immunity, although the immune response varies according to the worm species, and levels of exposure to the parasite.
The relative importance of the different GI nematodes differs with host age because of acquired immunity. The immune response to GI nematodes is directed at different stages of the parasite development and is a complex interaction of humoral and cellular immunity.
Normally, a protective host resistance develops against Nematodirus and Cooperia within one year. Ostertagia, Teladorsagia and Trichostrongylus spp. stimulate a slower immune response and are therefore sometimes seen in older stock (Vercruysse and Claerebout, 1997).
Interestingly a study conducted in Brazil found that animals challenged with Haemonchus placei three times a week for 25 days, then treated, and experimentally infected again with Haemonchus placei on day 35 displayed an intense immune response with low parasite establishment, compared to animals who were only challenged once with Haemonchus placei, demonstrating that the protective immune response to Haemonchus placei only develops when animals are repeatedly infected with this species (Santos et al., 2014). However, the same was not seen for Haemonchus contortus and which induces relatively poor acquired immune response (Adams and Beh, 1981) even after reinfection (Barger, 1988). This may explain why Haemonchus can also affect (and kill) adult sheep.
A vaccine against Haemonchus contortus in sheep, (BarbervaxTM) has recently been developed by the Moredun Research Institute in the UK and is licensed for Australia.
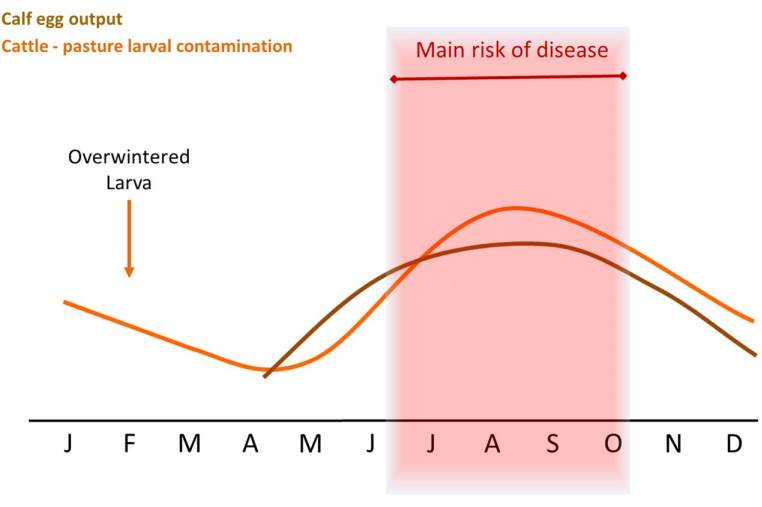
Another more recent study carried out in Sweden reported that calving season is a stronger determinant of worm burden in pasture-fed beef than the level of residual larvae in the field. They suggest this is because adult cows produce large amounts of feces at turnout which significantly contaminates the pasture, and early born calves are more likely to ingest more herbage, leading to higher risk of exposure to parasites (Höglund et al., 2013).
Immunity and parasite burden is also influenced by genetics, pregnancy, and nutritional state.
Genetics and PGE
Animals that withstand a heavy parasite burden and shed lots of eggs in their feces but continue to grow and perform are known as resilient animals. These animals have the ability to resist the pathogen and grow well regardless of worm burden often coping well without treatment. However, the danger with these animals is they are contaminating the pasture for the less-resilient animals.
Contrastingly, genetically resistant animals are those that are able to tolerate a pathogen by maintaining low worm burdens irrespective of infection pressures because they have enhanced immune responses which prevent adult worms establishing in their gut. The drawback is the strong immune response requires energy and can cause local gut mucosal changes resulting in weight loss and scours, affecting performance. Attempts are being made to identify the genes that encode parasite resistance, with the aim of identifying and selecting animals with superior parasite resistance or – controversially – even being able to develop transgenic animals with genes for parasite resistance, and economically productive traits.
The genetic basis of both resilience and resistance is complicated and depends on the fate of the animal as to which best suits a particular farming system. If breeding stock are resistant, they will be slower growing, but not infecting the pasture for their progeny. It could be beneficial for finishing stock to be resilient as they will continue to grow and perform well regardless of worm burden.
According to SCOPS (Sustainable Control of Parasites in Sheep), the biggest gains are being made by selecting rams that sire ewe breeds. If ewes are genetically resistant, their egg output during the periparturient rise and other times of the year will be lower than that of the average population, resulting in lower exposure of lambs.
In commercial flocks just selecting individuals with low egg counts will not lead to significant genetic progress.
Pregnancy and PGE

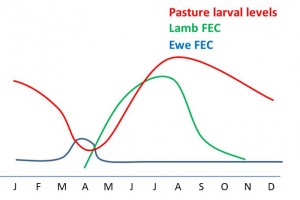
Ewes play a significant role in pasture contamination around lambing time due to a phenomena called Peri-Parturient Rise (PPR).
Peri-parturient rise (PPR) also known as spring rise, or post-parturient rise, is the name given to a large increase in fecal egg counts that occurs about 2 weeks before birth, and for about 6 week afterwards. It occurs mainly in ewes, goats, sows and to a lesser degree in cattle. It is thought to be due to the relaxation of the immune system in late pregnancy, allowing for parasite infection, or hypobiotic larvae to mature.
The late stages of pregnancy and lactation in ruminants necessitate an increase in their nutrient requirement. The grass quality around lambing is often poor and the inability of the ewe to meet nutritional requirements exacerbates PPR (Walkden-Brown and Kahn, 2002). Fecal egg counts during the spring rise are higher in animals bearing twins and triplets, compared to those with singles.
PPR is important in parasite survival as the large numbers of eggs passed onto the pasture at the same time as the numbers of new susceptible hosts also increases.
Nutritional Status and PGE
Nutrition availability and PGE can be considered from two angles. The first is linked to the damage caused by parasitic infection inhibiting digestion, and the second is linked to the immune response.
The damage caused by a parasitic infection in the gut will vary depending on larval challenge and worm species, and is influenced by the animal factors detailed above. GI nematodes influence nutrient availability in one of two ways, they suppress the hosts appetite reducing feed intake, or the worms damage the gut epithelium increasing the pH, which consequently reduces the breakdown and absorption of proteins and other nutrients negatively affecting the feed conversion efficiency.
Experiments using radio-isotopic markers have shown that protein losses into the gastrointestinal tract can be substantial, varying from 20 to 125 g protein per day in Teladorsagia colubriformis-infected sheep (Poppi et al., 1986).
It is fair to assume that in most cases improved nutrition will lead to improved ability to induce an immune response and resist disease, especially with gut parasites. Nutrition of the host impacts how well the immune system responds to larval invasion. Protein in particular is required as it provides the building blocks for antibodies and other molecules. It has been demonstrated that ewes fed a high quality diet, compared to ewes fed a poorer diet have a lower FEC during PPR, and higher levels of antibody and numbers of goblet cells in the small intestine (Beasley et al., 2012).
Mineral supplementation is thought to help the immune response. Swedish pelt lambs provided with access to a mineral supplement containing elemental copper, had significantly lower fecal eggs counts from multiple nematode species in the later part of the grazing season than those without access the supplement (Waller et al., 2004).
The supplementation of selenium may improve the humoral immune response and therefore reduce worm burden. This has been recently demonstrated in lambs infected with H. contortus but supplemented with selenium and copper (Fausto et al., 2014). Also lambs supplemented with Vitamins E had a lower burden of H. contortus (De Wolf et al., 2014). However, copper supplementation (Copper Wire Particles) was not shown to have the same benefits in first season grazing steers (Dimander et al., 2003).
Cobalt is known to play a role in immune functions implying that a deficiency could lead to a greater severity of parasite infection (Paterson and MacPherson, 1990). Calves deficient in cobalt and experimentally challenged with O. ostertagi had a higher worm burden and egg output compared to calves sufficient in cobalt (MacPherson et al., 1989).
Hypobiosis and PGE
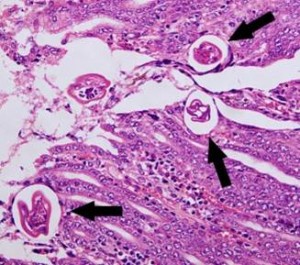
Sheep abomasum containing encysted hypobiotic L4 of Trichostronglus axei. Source: www.vet-parasitology.com/strongyloida.php
This is sometimes referred to as arrested development. The infective L3 are ingested by the host where they migrate to tissues and cease to develop until they are re-activated by a stimulus. The nature of this stimulus is still uncertain.
Hypobiosis is linked to pregnancy and the PPR and is significant in terms of parasite survival. In temperate climates the infective L3 usually undergo arrested development over the winter period, then re-activate around spring. Hypobiosis enables the nematodes to survive the winters and re-emerge in spring where they mature to adults and then contaminate the pasture with eggs.
Hypobiosis also gives rise to type II ostertagiosis and type II teladorsagiosis. More about each of these conditions can be found on there respective pages.
Environmental Factors and Nematode Survival
The free-living stages of GI nematode development are often overlooked by farmers yet they are imperative to enable parasitic infection. The free living stages can be sub-divided into 2 stages both of which are largely dictated by climate:
- The first stage is egg development to infective L3
- The second stage is L3 survival.
Climate influences development and survival of larva on the pasture, and the distribution and migratory behavior during the free-living stages. The main factors are temperature and humidity but also sunlight (UV light) can increase mortality.
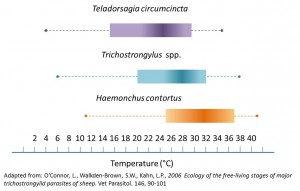
Optimal development conditions for Teladorsagia circumcinta, Trichostrongylus spp. and Haemonchus contortus.
Extreme heat and cold are detrimental to development and survival, but are tolerable within certain limits (Morgan and van Dijk, 2012). Broadly speaking eggs from the common ruminant GI nematodes found in the UK will develop above a threshold of 39.2-41°F except for Haemonchus which is 46.4°F (O’Connor et al., 2006). As temperature increases, so does the rate at which the eggs develop into infective L3. Increasing warmth not only accelerates development it also increases the rate of L3 mortality. This is to some extent due to the inability of infective L3 to feed (only the pre-infective stages of L1 and L2 feed), and the increased metabolic demand due to increased temperature causing them to perish quicker at higher temperatures.
Moisture from humidity, rainfall, the soil or the fecal pat is required for L3 development and survival and to enable the L3 to migrate out of the fecal pat but is not needed for onward migration onto grass (van Dijk and Morgan, 2011). The effect of rainfall on larval dispersal is also important as a drop may transport larvae as far as 35″ from the dung pat (Stromberg, 1997). Eggs and Larva rarely survive in arid climates.
Under optimal conditions of moisture and temperature Ostertagia ostertagi will reach the infective stage in about 5-6 days (Stromberg, 1997).
Sunlight and UV rays increase L3 mortality (Dijk et al., 2009). Desiccation of L3 under lab conditions at 77°F with continuous exposure to UV and 100% humidity, Nematodirus battus 2.5 days, Teladorsagia circumcincta 3 days, and Haemonchus contortus 5 days. Under natural sunlight more L3 survived, but the L3 in the shade survived twice as long (Dijk et al., 2009).
All of these climatic factors combined make up the seasonal patterns, hence, most clinical cases of nematode infection occur in growing stock in the second half of the grazing season when there is gradual build-up of infective L3 on the pasture.
A good understanding of what drives parasite biology is needed in order to put together a control strategy that does not solely rely on anthelmintics.
Sustainable parasite control strategies require knowledge of seasonal larval availability and the climatic requirements for eggs embryonation and hatching, larval development and survival. Different nematodes have evolved to withstand different climatic factors. For example Teladorsagia circumcinta is very tolerant of cold conditions and able to survive below 41°F, whereas Haemonchus contortus is sensitive to cold but can however, survive well in hot climates. More information about each of the ruminant GI nematodes of veterinary importance can be found on their individual pages:
- Cooperia onchophora
- Haemonchus contortus
- Nematodirus battus
- Ostertagia ostertagi
- Teladorsagia circumcincta
- Trichostrongylus spp.
Nematode infections are almost always a mixture of species.
Farm Management Factors and PGE
The grazing system and pasture management impacts on levels of pasture contamination, and therefore worm burden in stock. Different grazing management strategies can be used to minimize larval challenge by targeting, or avoiding the obligatory free-living stage on the herbage to provide ‘safer’ pasture for the stock to graze. Click on each link to skip to the relevant sections:
- Mixed grazing
- Alternating stock
- Rotational and evasive grazing
- Extensive grazing and stocking density
- Cutting and reseeding
- Pasture composition
Control and Prevention of PGE
Parasitic gastrointestinal roundworms are ubiquitous on farms. Parasite control programs are not there to eliminate worms fully but to reduce the burden and minimize the negative impact on health, and improve production. Farmers must integrate as many different control methods that are practically and economically feasible as the only way of ensuring long-term sustainability (Waller, 2006).
Unfortunately there is no silver bullet solution for eradicating worms from the farm, but instead there are a variety control measures that can be applied on farm to minimize challenge, and control worm burden. The control measures described below must be used in combination as no single measure works in isolation. Nevertheless it should not be overlooked that any control measure in which there are GI nematode that survive is subject to evolutionary advancement in which these survivors will produce descendants that may be able to endure unfavorable conditions, enforced by the control measure.
Before any approach to control is attempted it is good to know what you are dealing with and a simple way to do this is through Fecal Egg Counts, and an Anthelmintic Resistance check.
Fecal Egg Counts
Fecal egg counts (FEC) are a diagnostic tool in which the number or worm eggs per gram of feces is calculated, and can be used as an indication of parasite infection. FECs are conducted using either the McMaster technique or FECPAK which is a modified version of McMaster developed for farmers to use in the field.
FECs are a good method for monitoring GI nematode infection, and potential pasture contamination especially when attempting a new control approach. Furthermore they are often used by farmers as an indication to treat over a pre-determined threshold.
In cattle FECs are a useful measure of concentration of worm eggs being excreted onto the pasture, and are frequently used by researchers and clinicians to test for , however FEC is not necessarily consistently correlated to worm burden, or pathology. More can be read about this in the COWS technical manual: Controlling PGE in cattle.
This does not work for Nematodirus – please refer to Nematodirus page to find out why.
FECs are not necessarily correlated to worm numbers or to the severity of parasitic disease. They can be used post-treatment to help determine the effectiveness of an anthelmintic treatment. Failure to achieve at least 95% reduction of faecal egg counts is indicative of worm resistance. See anthelmintic resistance.
FECs are not flawless and should only be used alongside other diagnostic methods to determine a clinical diagnosis. The advantages and limitations of FEC s are outlined in the table below.
| Advantages | Limitations |
| Can be used to determine whether to treat or not | Zero eggs does not mean no disease* |
| Used to test the effectiveness of treatment | The correlation between worm burden and egg output is low |
| Assess pasture contamination | Not a definitive diagnosis |
*For some worm species like Nematodirus, the clinical signs of PGE are caused by the immature larvae in the gut.
Anthelmintic Resistance
Anthelmintics are anti-parasitic drugs used to treat worms, fluke and other helminth infections. Anthelmintic resistance (AR) occurs when the worms are able to tolerate a drug at its normal dose, and this ability is passed on to their offspring. The drug is essentially losing its effectiveness (efficacy). Reports of anthelmintic resistance in worms are widespread and, given reliance on drugs for worm control on farms, AR threatens the viability of the livestock industry worldwide. Resistant worms are an increasing concern, so far more in sheep than in cattle, and there is an urgent need to alter control strategies to take account of this. The most efficient way to limit the increase of anthelmintic resistance is the reduction of the selection pressure by drugs, and optimal timing to maximize their efficacy (Silvestre et al., 2002).
How do you test for resistance?
Resistance on farm is detected using a “Drench Check” or a Fecal Egg Count Reduction Test (FECRT), full details of which are on the SCOPS website or COWS. In short, FECs are carried out on a number of individuals pre-treatment, then the FECS are repeated a number of days (dictated by the drug used) post-treatment. Comparisons of the egg counts give an indication of drug efficacy and where this has fallen below 95%, anthelmintic resistance is suspected. A simpler version of the drench check uses pooled FEC from a minimum of 15 animals before and after treatment (as dictated by the drug used). This is less precise, but considerably cheaper and if conducted routinely should give an early indication of a developing problem.
Resistance levels evolve and tests should be carried out at least every 2 years. Ideally, drench checks should be incorporated into routine worming protocols. The rate at which resistance develops is dependent on many factors including the species of worm being targeted and the number of worms in refugia. The refugia populations are the worms that do not come into contact with the drug during treatment and therefore remain susceptible and include larvae on the pasture, worms in untreated animals, and inhibited larvae inside the host.
Previously the advice was to dose stock, then move them onto clean pasture. The problem with this is any worms that survive the treatment are considered drug resistant, and these will then reproduce creating more worms that are able to survive the dose, hence anthelmintic resistant populations of worms will increase. Maintaining a refugia population of worms susceptible to will slow this process.
Target select treatment, i.e., only treating those animals that need treating will maintain refugia. Animals can be selected from treatment based on:
- Poor weight gain in young stock
- Dag score for sheep
- Dairy cattle – milk ELISAs
- FAMACHA for Haemonchus
Changing the class of anthelmintic used is recommended to preserve the efficacy of the drugs. This can involve using benzimidazoles when the main risk is Nematodirus, or using the newer classes at certain times if resistance to other groups has been confirmed.
Incorrect use of a drug or under dosing enables resistant populations to grow faster. See SCOPS or COWS guidelines on the recommended dosing procedures.
Other Control measures
Practical grazing management systems require effort, forward planning and are initially difficult to quantify, however the following strategies have shown some success under experimental conditions:
- Mixed grazing
- Alternating stock
- Rotational and evasive grazing
- Extensive grazing and stocking density
- Cutting and reseeding
- Pasture composition
Mixed Grazing

Mixed grazing is co-grazing susceptible and non susceptible stock. Most parasites are host species specific, in that cattle parasites do not infect sheep and vice versa.
Mixed grazing is a diluting strategy in which susceptible animals are grazed with non-susceptible animals. The non-susceptible animals can either be older stock of the same species or stock of a different host species. The common mixed grazing species are cattle and sheep or sheep and horses. The theory behind mixed grazing relies on either older stock previously being exposed to parasites and having sufficient immunity or on the parasite species being host specific – i.e., cattle parasites only being pathogenic to cattle.
Mixed grazing is not recommended for sheep and goats, as many parasites jump between those species, intensifing the problem.
An example of mixed grazing is cattle grazing with post-weaned lambs, the cattle will by default be ingesting some of the L3 infective to the lambs, thus reducing the risk to the lambs. Mixed grazing will also reduce the rate of pasture contamination by worm eggs as susceptible animals grazed with non-susceptible animals in comparison to the field being stocked with the same number of just susceptible animals.
When solely looking at mixed grazing and animal performance researchers in Wales found that cattle mixed grazing with ewes and lambs from May to July, then just with weaned lambs from July to October had the fastest growth rate of lambs (Fraser et al., 2007; Marley et al., 2006). Similarly Jordan et al., (1988) also looked at the effect on cattle and found that mixed grazing sheep and cattle resulted in reduced sheep parasitism, and increased productivity of lambs, but at the expense of increased cattle parasitism and reduced productivity of the calves. These results suggest that this mixed grazing system suited sheep and not cattle, so when mixed cattle are disadvantaged, making it difficult to maintain cattle productivity.
However farmers should be cautious when mixed grazing as it can select worms that are non-host specific, such as Trichostrongylus axei. If T. axei is a major concern on your farm mixed grazing should be avoided as T. axei has evolved to infect several ruminant host species. Also there have been occasional reports of Ostertagia ostertagi infecting sheep and goats (Barger, 1999).
Other diseases to consider with mixed grazing are liver fluke, leptospirosis, Johne’s and possibly mange in cattle. In the case of Johne’s, both sheep and cattle can be infected so if a farm is trying to control Johne’s in a dairy herd, they should avoid mixed grazing.
Alternating Stock
Much like mixed grazing, sequential or alternate grazing is a control strategy that exploits host-specificity, by which parasites pathogenic to one species do not infect alternative hosts, or are less pathogenic. The alternation of sheep and cattle in Wales, with cattle grazing from May to July, then post weaned lambs from July to October was most effective in reducing worm burden in both species and cattle mixed grazing with ewes and lamb May to July, then just with post-weaned lambs from July to October had the fastest growth rates of lambs (Marley et al., 2006).
However, researchers in Scotland carried out a 4-year study investigating the effectiveness of alternate grazing sheep and cattle in the control of bovine PGE. They found that annual sequential grazing of cattle and sheep, which worked initially for two consecutive years, but by the third grazing season had lost its effect, concluding that sequential grazing was not beneficial in controlling PGE in cattle long-term (Bairden et al., 1995). They did however recommend a longer resting period of a 3 year cattle/sheep/crop cycle.
This grazing strategy is not recommended for farms who suffer from Haemonchus or Nematodirus as they can infect both sheep and cattle.
Rotational and Evasive Grazing
Rotational grazing is management intensive and involves increased capital costs due to fencing, labor and water supply but it does allow farmers to match nutritional demands of livestock with the availability of forage.
Rotational grazing is more suitable in tropical climates where larval (pasture) development and survival times are short and is less achievable in temperate climates as substantial declines in pasture infectivity may take 3 – 9 months depending on the larval species (Barger, 1999). Rotational grazing involves the sub-division of pasture into smaller paddocks and each paddock is grazed for a short time, then rested. For instance a whole field may be sub-divided into 15 paddocks and each paddock is grazed for one week, then rested for 14, making the rotation time 15 weeks.
The time animals spend in each paddock will be dictated by sward height and grass cover.
In temperate climates farmers should be careful with rotational grazing as under certain conditions it can intensify the problem. For example first season grazers shedding eggs into paddock 1 and rotational graze on a weekly cycle around 4 paddocks (i.e., 1 week per paddock), paddock 1 will be left untouched for 3 weeks during a warm wet summer, these undisturbed eggs will likely have to developed into infective L3 ready for when they return.
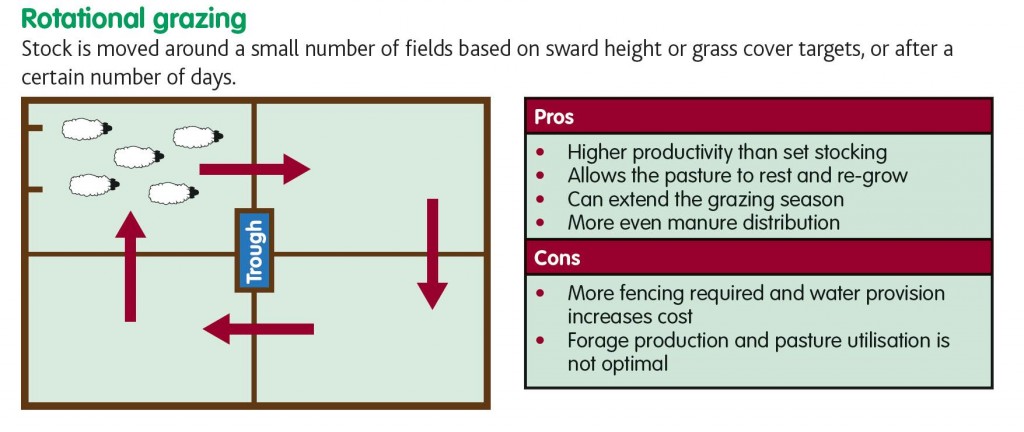
Rotational grazing is beneficial in tropical climates for controlling worm burden, but it cannot be relied on in temperate climates. In fact if the rotation is too short, it can actually amplify the issue. However rotational grazing is good in terms of grass utilization and productivity.
This diagram has come from Eblex Beef and Sheep Manual 8 – Planning grazing strategies for better returns.
The sward may be compromised during rotational grazing so a balance of pasture infectivity and productivity needs to be met. The pasture resting period must be long enough to allow sufficient time for pasture re-growth, and also for any infective larvae to die off. Different worms die at different rates depending on seasonal influences so it is useful to understand which parasite species are present on your farm if considering rotational grazing. In theory the longer the resting period the better as the L3 will starve and die, however if the rotation is too long, the sward may become unfit for grazing.
Evasive grazing is avoiding high risk pastures at critical times. Herbage larval levels are highest during the second half of the grazing season, so avoiding highest risk pastures at these times is an evasive method to reduce larval challenge in susceptible stock.
In the Netherlands evasive grazing by late turnout onto mown pasture (aftermath) is performed on approximately 70% of Dutch dairy farms, but has been proven to be more complicated in sheep systems, largely due to the ability of Haemonchus to build up high pasture infectivity in just over 2 weeks (Eysker et al., 2005).
Extensive Grazing and Stocking Density
Extensive grazing is often carried out in the uplands and is one that simulates nomadic livestock systems. Animals have access to one area of grassland and are set stocked throughout the whole grazing season. Extensively grazed grassland creates a diverse sward structure rich in plant and invertebrate biodiversity, and due to the size of the pasture stocking densities are low and there is more opportunity for animals selectively graze. Increasing stocking density leads to increased parasitism in livestock and this has been demonstrated in a number of studies (Brown et al., 1985; Southcott et al., 1967) probably because the animals are forced to graze closer to fecal pats (Thamsborg et al., 1996).

Evasive grazing takes advantage of large expanses such as moorlands, which in comparison to fields will have low stocking densities.
Pasture Management: Cutting and Reseeding
A group of researchers in Sweden showed that efficient and sustainable parasite control of first season grazing cattle was possible to achieve without the use of anthelmintics by using turnout pastures that the previous year had been grazed by older cattle, in combination with a mid-July move to aftermath leys (Dimander et al., 2003). The older stock should hoover up the infective larvae, then the aftermath provides animals with a pasture that is free of fecal contamination.
Moving cattle monthly to fresh aftermath has been shown to be effective at reducing worm burdens. In one study, the cows were turned out in May, and moved onto aftermath in July, August and September, then were housed in October. The group that were moved had a significantly lower worm burden than those only moved once or twice (Eysker et al., 1998).
The rationale for re-seeding lies mainly in the notion that larvae live on herbage or within the fecal pat (depending on their development stage), and by plowing them in to the soil, they would perish. However, it is difficult to find research to support this theory, even though there is a lot of studies that ‘clean’ pastures for parasite research by a complete plow and re-seed, and pasture infection with Nematodirus spp survived plowing and reseeding, though in very small numbers (Boag and Thomas, 1975).
Nevertheless, tillage has shown to reduce the levels of free-living larvae and soil-dwelling nematodes (Lenz and Eisenbeis, 2000) so it is reasonable that this concept can be applied for GI nematode larval survival.
Pasture Composition
Birdsfoot trefoil, lotus, sulla and sainfoin contain condensed tannins while, chicory contains sesquiterpene lactones, both have been shown to have potential anthelmintic properties. Marley and colleagues found that chicory had no effect on egg hatchability but significantly affected the development / survival of infective larvae in sheep feces (Marley et al., 2003a). In another study the same researchers reported that lambs grazing chicory did not have significantly lower FEC than lambs grazing other forages but these lambs were found to have fewer total adult worms in their guts than lambs grazing ryegrass/white clover (Marley et al., 2003b). There is little evidence on the effect of chicory in cattle: a recent study found no difference in Belgian Blue steers grazing a pasture containing chicory and a pasture without it, however, both groups were treated routinely three times with an avermectin pour on wormer, minimizing any possible difference (Marley et al., 2014). For more on the anthelmintic properties of plants, see Sheep Nutrition.
The inconsistent outcomes from studies investigating bioactive forages is related to the many factors which affect the production of the secondary metabolites including climate, soil type, other species in the sward, season, soil nutrient availability, grazing and cultivations.
Approaches to control should encourage the development of host immunity, keep your stock healthy and disease free, monitor what is happening on your farm and allow you to respond accordingly, and to maintain good quarantine protocol for new stock. Veterinary, agronomical and other professional advice should be taken to create a farm specific control plan.
Treating PGE
Many factors should be considered before deciding when to dose animals, including age of animals, grazing and dosing history, performance/condition and clinical signs of scour/illness. One of the most practical recommendations to control anthelmintic resistance is the specific targeting of anthelmintic dosing to when animals actually need it (McCoy et al, 2005).
The majority of currently available anthelmintics used to control parasitic nematodes of cattle and sheep belong to only three main groups, the benzimidazoles, imidazothiazoles and the avermectins/milbemycins. In sheep there are two new groups: Monapantel (Zolvix) and Derquantel and Abamectin combination (Startect). The successful implementation of control programs that limit the development of anthelmintic resistance depends partly on effective detection and monitoring (Taylor et al, 2002).
See the different GI nematode pages for specific treatment options.
Good Practice Based on Current Knowledge
-
- Adopt a system of regular and strategic monitoring using fecal egg counting
- Use forage crops with high quality protein, such as chicory, plantain, sainfoin and lucerne, strategically, to increase the resilience of the youngstock to helminthiasis and possibly increase their immunity
- Lower stocking density
- Use FECs to monitor worm burden and reduce the frequency of drenching
- Avoid importing resistant worms by following the SCOPS or COWS quarantine guidelines
- If treatment is required:
- Adopt a rotation of anthelmintic classes
- Ensure the use of the correct dose rate
- Calibrate the drenching equipment
- Dose to the heaviest in the group
- Fecal samples should also be taken after treatment with an anthelmintic to test the efficacy of the drench used and establish whether there is anthelmintic resistance on the farm

American Consortium for Small Ruminant Parasite Control
For more information on the sustainable control of internal parasites of sheep and goats, we recommend visiting the website of the ACSRPC www.wormx.info. The site contains a range of excellent resources, including videos, very informative fact sheets and technical guidance, scientific papers and other sources of knowledge and advice


 British English
British English



Comments are closed.