Cattle Diseases
Lungworm in Cattle
Also known as: Dictyocaulus viviparus, Parasitic Bronchitis, Husk
Epidemiology of Lungworm in Cattle
Female worms in the host’s lungs produce eggs containing fully developed larvae (See lifecycle below). They hatch in the bronchi, are transported up the wind pipe, swallowed and are passed in the feces. They can develop to infectivity in approximately 1 week (Dijk, 2004), which is much faster than most of the eggs of gut worms (gastrointestinal nematodes) .
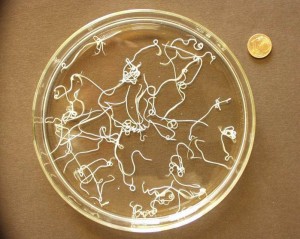
Lungworm in a petri dish and a the 1 cent euro coin in the top right corner of the image, to illustrate how big the adult worms are.
Larvae are dispersed to the area around fecal pat by the common fecal fungus Pilobolus. They are propelled into the air on discharge of the sporangium (Eysker, 1991; Eysker and De Coo, 1988). Infective larvae can remain viable in soil as well as on pasture over the winter (Duncan et al., 1979; Oakley, 1979). Larvae are very sensitive to dehydration, therefore, most of them die after 2-3 weeks in the summer if conditions are dry, although survival in autumn can be much longer (Dijk, 2004). Differences in temperature and rainfall can dramatically alter the amount of infective larvae present on pasture, although optimal conditions can produce a rapid increase in pasture larval numbers.
When ingested the infective larvae migrate to the lungs, provoking a strong immune response that is short-lived, unless the animal has previously been expose to the larvae within the past six months. D. viviparus can grow for a few millimeters in length to centimeters in about 3-4 weeks.
A small number of adult worms and hypobiotic larvae can survive and overwinter in infected animals. In contrast to gut worms (gastro-intestinal nematodes), silent carriers of lungworm are the most important source of larval contamination of pasture rather than larvae surviving at pasture over winter (Dijk, 2004).
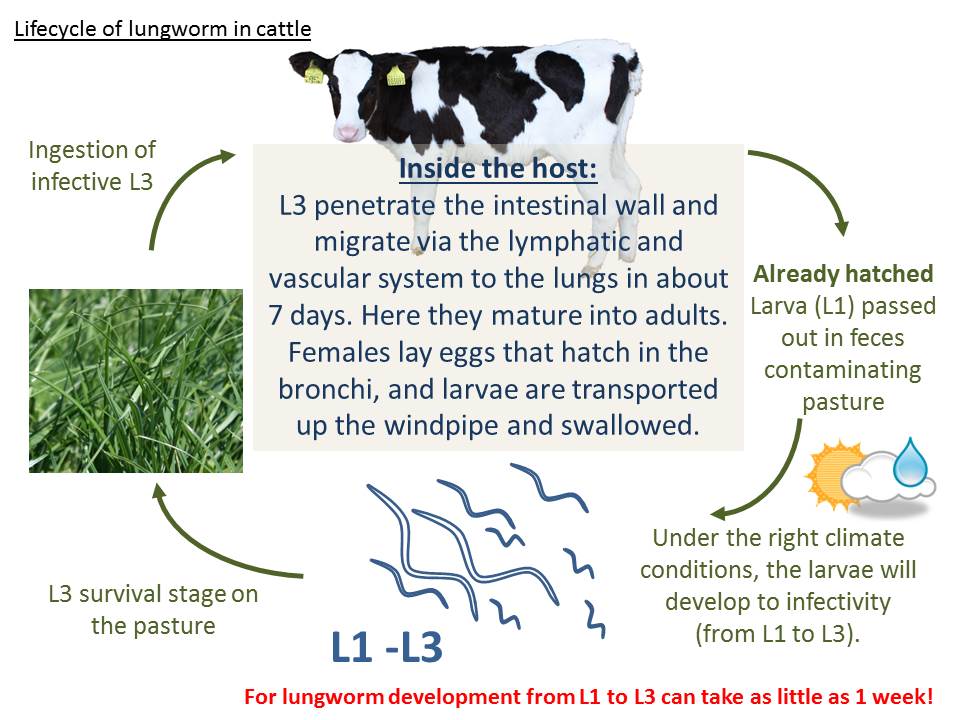
The lifecycle of cattle lungworm (Dictyocaulus viviparus) is similar to that of ruminant gut worms, except instead of unembryonated eggs being shed in the feces, first stage larva (L1) are shed, and they can develop to infective larva (L3) in as little as 1 week.
David (1997) identified several risk factors from a survey of 32 mainly dairy herds (10-210 cattle) in England with a history of lungworm outbreaks. These included:
- Failure to vaccinate a group of animals on an endemically infected farm
- Susceptible groups of animals joining an endemically infected herd
- Reintroduction of infection from introduced livestock
- Young stock grazed away or housed in their first year (David, 1997)
However, the epidemiology of lungworm remains less predictable compared to gastrointestinal nematodes. In recent years, increasing numbers of clinical cases of lungworm have been reported in adult dairy cows (Dijk, 2004). This is thought to be due to failure of development of immunity, either through reduced vaccine use or due to increased reliance on long acting anthelmintics, which prevent development of larvae to a stage when lasting immunity is stimulated. Cases of clinical lungworm have even been reported in housed calves (Crawshaw and Smith, 2003).
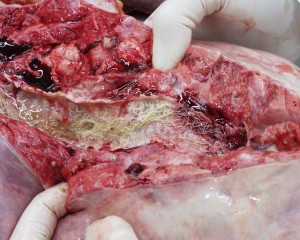
Lungworm in the bronchi of an infected calf. The lung capacity of a cow is only 4% of its body weight, which in comparison to the lung capacity of humans (Which is about 10% of their body weight), is not much, so damage or obstruction in the airways is much more significant in cattle.
Immunity consists of two phases:
- Short-lived immunity: Resistance to the initial colonization of the lungs and establishment of infections (i.e., larvae reaching lungs). In order to migrate to the lungs, the larvae need to travel via the lymphatic system, and are trapped in the mesenteric lymph nodes. This immune response weakens rapidly over time and only lasts for about 6 months
- Long-lived immunity: Prevents the development and survival of adult worms in the lungs (Dijk, 2004). This immune response lasts for over 2 years. Re-infection each grazing season is necessary to maintain a strong immunity, which in most herds occurs through exposure to low levels of larvae from adult carrier cows
Reinfection syndrome: However, even in the face of immunity, a massive infestation of larvae can penetrate the lung faster than the immune system can respond and lead to development of “Re-infection husk”. In theory, although resistance to invasion of larvae can develop when animals are under anthelmintic treatment, it is unlikely that it is sufficient to stimulate long-term immunity (Dijk, 2004; Höglund et al., 2003; Taylor, 2000).
Clinical signs of Lungworm Infection
Clinical signs may vary from occasional coughing to severe respiratory distress, and symptoms reflect the number of infected larvae ingested during a relatively short period. Typically, the peak incidence of clinical cases occurs in late summer and early autumn (August-October) though can persist until December (Dijk, 2004). In adult dairy cattle, milk drop and reduced fertility may be a typical clinical sign. By the third week, severely affected cattle do little else except stand in a characteristic head-extended position with rapid shallow breathing and frequent coughing (Taylor and Andrews, 1992). Harsh lung sounds are heard over the ventral lung field. There may, or may not be a patent infection (larvae in feces) when clinical signs are seen. Clinical disease in adult dairy cows can result in severe losses, mainly in terms of reduced milk production, reduced fertility and potentially even the death of the cow.
Control and Prevention of Lungworm in Cattle
Mixed results have been obtained from rotational grazing to control lungworm. D viviparus larvae can last as little as 4 weeks in short swards on hot sunny conditions, but in cooler years, with longer herbage they can survive >12 months. Rotation of grazing once a week over 6 paddocks during the grazing season prevented the occurrence of severe lungworm infections and permitted the development of immunity to Dictyocaulus viviparus, but rotation of grazing once every two weeks failed to control lungworms (Eysker et al., 1992, 1993a). Simply put, rotational grazing is unreliable, as its success is largely dependent on the climate and sward. Also rotational grazing does not lead to the development of immunity to gastro-intestinal parasites and therefore cannot be recommended as a general approach to parasite control (Dijk, 2004).
There has been research into biological control in recent years (Hertzberg et al., 2002). Henriksen (1997) found an 86% reduction in larval release from fecal cultures by nematode-trapping fungi (Henriksen et al., 1997). Laboratory investigations of Aphodius beetles (Scarabaeidae) as biological control agents of lungworm have been conducted by researcher in Ireland (Gormally, 1993). Unfortunately, none of these methods turned out to be practical in commercial herds.
Unlike the majority of nematode infections, there is a vaccine against lungworm (Husk) in cattle. It is a X-radiated larval vaccine that stimulates initial immunity, but in order to develop a strong immunity, the animal has to undergo an infection with larva at pasture also (Dijk, 2004)
Generally there are two major methods available:
- Vaccination
- Anthelmintic suppression of infection.
Vaccination
Immunity can be stimulated by the use of live vaccines (Bovillis Huskvac, MSD Animal Health), which take the form of two doses of 1000 infective L3 larvae irradiated by gamma-irradiation. Vaccination is aimed at first season grazing animals, but can also be safely used in pregnant older animals if required (Holzhauer et al., 2005). Although the vaccine induces good protection against clinical disease, it does not completely prevent all worms from natural infection from completing their lifecycle, indeed, development of immunity is dependent on exposure to larvae at pasture following vaccination. As a result the larval pasture levels should be remain low on pasture grazed by vaccinated cattle. On farms where calves have suffered lung damage due to viral pneumonia, vaccination may be inadvisable because of the possibility of exacerbation of the existing lesions. With the unpredictable epidemiology of lungworm and limited efficacy of strategic grazing protocols, vaccination is often the most reliable and cost effective method of control on farms.
Other limitations are the short shelf-life of the vaccine and the short window of availability. This makes it difficult to introduce a vaccination program in spring born beef calves.
Anthelmintic treatments
Regular anthelmintic treatments are potentially problematic with this as some animals will build up insufficient immunity in the first grazing season and may become ill second or later years (Andrews and de Wolf, 1994; David, 1993; Eysker et al., 1993b; Jacobs, 1993; Taylor et al., 1997; Wilkinson, 1992). Similar findings have been made when using the Michel’s `dose and move’ system (Eysker et al., 1995, 1997). Suggested combination approaches of vaccination and dosing include:
- Turn calves out onto pasture following vaccination in spring/early summer, then dose with an anthelmintic and move to aftermath or clean grazing in mid-July;
- Turn calves out as before but move calves each month from the start of July to aftermaths or grazing grazed by adult cattle or sheep (Dijk, 2004). However, this may possibly select for anthelmintic resistance, if calves always return to clean grazing after treatment.
There is some debate as to establishing the threshold for treatment of cases of parasitism (Thamsborg and Roepstorff, 2003; Vercruysse and Claerebout, 2001). A therapeutic threshold identifies animals with clinical disease, a production-based threshold measures effects on productivity and a preventive threshold is used to predict and control future infection. In sustainable farming systems, it may not be appropriate to merely consider production at the expense of immune development, although it should be remembered that widespread clinical disease is unacceptable (Thamsborg and Roepstorff, 2003).
Treating Lungworm in Cattle
In order to kill all stages of Dictyocaulus viviparus, anthelmintic treatment is essential. All anthelmintic groups will target lungworm. However, you should follow the COWS (Control of Worms Sustainably) guidelines to ensure effective treatment. Severely affected animals may require antibiotic treatment to control secondary bacterial pneumonia and, if anorexia is present, rehydration with electrolytes. Non-steroid anti-inflammatory agent should also be used as a support therapy. The worst affected animals should be housed, especially in poor weather conditions. Treatment may initially exacerbate the signs due to mortality of worms in the airways. Additionally, despite treatment, some cases may relapse a few weeks after treatment due to the severity of the lung pathology.
Good Practice Based on Current Knowledge
Methods that can be used to prevent lungworm outbreaks are:
- Low stocking rates
- Closed herd policy or quarantine and treatment of introduced stock
- Do not run young calves with older calves
- Avoid lush wet pastures or paddocks with swampy areas if possible at times of high risk
- Use rotational grazing if possible, moving at weekly intervals, followed by adult immune cattle
- Blood samples can be analysed for antibody to D. viviparous (van Dijk, 2004) and fecal larvae counts can be used to assess exposure from early summer through the grazing season – however, a negative fecal result is not proof of absence of disease, especially in adult cattle with mild symptoms larvae are rarely found, but they do respond to anthelmintic therapy
- Vaccination may be required on farms where the disease is endemic. Vaccinated animals need to be exposed to parasitic challenge following immunization to ensure the development of adequate immunity.


 British English
British English


Comments are closed.