Cattle Diseases
Neospora caninum in Cattle
Neospora caninum is a protozoan parasite primarily causing abortions and reproductive problems in cattle and disease in canids (mainly dogs, but also foxes, coyotes, dingoes and grey wolves). Calves that are infected in utero that do not abort may be born weak, underweight and with neurological symptoms such as ataxia, decreased reflexes and exophthalmia (Reichel et al., 2014). However, they can appear normal but are more likely to abort later in life. Transmission of N. caninum is regarded as either vertical or horizontal. Horizontal transmission is from animal or group to another i.e., dog to dog, or dog to cow, or cow to dog (dog eating meat or placenta) and vertical transmission is from one generation to another i.e., dam to calf transplacentally (See disease transmission diagram below).
Impact on Production
Control and Prevention
Treatment Options
Good Practice
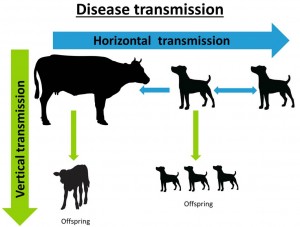
Neospora caninum is spread either vertically from one host to another (i.e., dog to dog, or dog to cattle), or horizontally from one generation to another (i.e., dam to calf). Vertical transmission of N. caninum is considered to be the principle route of infection in cattle.
Dogs (canids) are definitive hosts to N. caninum, this means they are required in order for the parasite to complete its whole lifecycle. Oocysts are shed in the faeces of an infected dog, and after as little as one week under the correct climate conditions they develop (sporulate) and become infective. Dogs can shed infectious oocysts for a variable period of time after infection and it is unclear how long these survive in the environment. These sporulated oocysts are thought to contaminate feed and water supplies of cattle. Infection from dog to dog via oocysts in faeces, or from dog to cattle via the same method is horizontal transmission.
When a cow becomes infected from dog faeces, the infection spreads (as tachyzoite) to other tissues in the cow, and if pregnant to the placenta, where damage to the placenta or vertical transmission through the placenta to the foetus can occur. Vertical transmission of N caninum is considered the principle route of infection in cattle, with the same cow able to pass infection to multiple offspring. Abortion may be a result of both the primary damage and the immune mediated inflammatory response of the cow (Maley et al., 2003). As a result of the immune response generated, the tachyzoite can transform into bradyzoites (a slowly dividing ‘dormant’ stage). They remain latent until the immune system of the cow is suppressed, when infection then can recrudesce (Haddad et al., 2005). Bradyzoites in tissue cysts can be consumed by dogs and then complete the life cycle of the parasite.
Additional hosts of N. caninum also include other wild canids such as foxes, with evidence of infection in goats, sheep, horses, deer, badgers, polecats, ferrets, and mink implying that wildlife may be a potential reservoir of infection (Bartley et al., 2013; Gondim et al., 2004; Haddad et al., 2005)
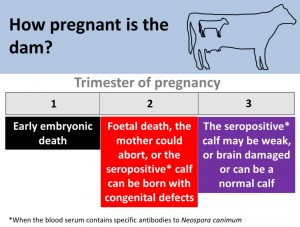
The stage in a dams pregnancy that she is first infected with Neospora caninum is crucial in defining the calf’s outcome. The calf will always be born seropositive but often with congenital defects.
The outcome of new infection or recrudesce of infection in a cow depends on the stage of pregnancy. In a non-pregnant animal, infection results in seroconversion and persistent infection. Although seropositive animals may conceive at the same rate as seronegative animals (López-Gatius et al., 2005), infection/recrudesce in the first trimester of gestation may result in early embryonic death. Infection/recrudesce in the middle trimester can result in abortion or the birth of a weak or brain damaged live calf (characterised by incoordination), whereas infection in the last trimester will result in a seropositive weak or brain damaged calf or a normal calf. It is an important feature of the epidemiology of this condition that, apparently healthy calves can be born to infected dams. These calves are however, congenitally (vertically) infected and are seropositive to N. caninum antibodies. Due to the greatest risk of abortion occurring in mid to late gestation, pregnancy diagnosis only undertaken before 5 months in endemically infected herds may lead to an overestimate of the number of animals that are due to calve (Weston et al., 2005).
There is some suggestion that probability of vertical transmission may decrease with age of dam (Haddad et al., 2005). The incidence of horizontal transmission is generally low. However, specific farms may undergo abortion storms attributed to N. caninum, which may be through the sudden increase in the incidence of horizontal transmission (infection from contaminated feed and water with canine faeces containing oocysts), although in many cases, the point source in unknown (Crawshaw and Brocklehurst, 2003; Dijkstra et al., 2001; Sager et al., 2005). The presence of canines on a farm are a risk factor for infection in cattle. Recently, N. caninum DNA has been reported in bovine semen, which may act as another route of infection into herds (Ferre et al., 2005), although there is no evidence of horizontal transmission yet from infected rams to ewes (Syed-Hussain 2013)
The prevalence of infection in the UK was estimated at 18% of aborting dairy cattle and 6% of normally calving cattle in 1997 (Davison et al., 1999), but is unknown in beef herds. Reports from Canada estimate herd level N. caninum seroprevalences of 5-25% in dairy herds and 6-9% in beef herds (Haddad et al., 2005) and in Sweden of 6-65% in dairy herds (Frössling et al., 2005). The prevalence of N. caninum infection of UK dogs in unknown. A bulk milk antibody survey in UK dairy herds carried out in 2012 found evidence of neospora in 51 % of herds (www.checs.co.uk)
The risk factors for infection are largely unknown, although evidence suggests that close contact of cattle with dogs and high stocking densities may be a risk factor (Dijkstra et al., 2002a, 2002b; Otranto et al., 2003). Whilst transmission by dogs has been considered of relatively minor importance, a Dutch study showed that seropositivity to N. caninum in farm dogs was strongly correlated with a high prevalence of N. caninum antibodies in the cattle (Wouda et al., 1999). Dogs that have close contact with cattle and that are fed raw bovine meat are at greater risk of being seropositive (Kramer et al., 2004) with dairy or beef farm dogs having a much higher seroprevalence of N. caninum infection compared to urban dogs (Antony and Williamson, 2003; Basso et al., 2001). Management factors or concurrent diseases that lead to immunosuppression may also be important (Reichel and Ellis, 2002).
Diagnosis of Neospora caninum
An ELISA (Enzyme-linked immunosorbent assay) commercially available and used routinely to test serum samples for Neospora-specific antibodies. The ELISA results are expressed as a percentage of a positive control included in each test (so-called per cent positivity [PP] values): results are negative if less than 20 PP; inconclusive if between 20 and 25 PP, and positive if greater than 25 PP. All serological results should be interpreted with caution as antibody levels have been reported to fluctuate (Fischer et al., 2003; Sager et al., 2001). Consistent results from multiple tests should ideally be used if making decisions on culling or re-serving (Haddad et al., 2005). Typically, in infected animals, antibodies are highest in late pregnancy or soon after calving or aborting. Infected yearlings may test negative, which has implications for testing bought in animals.
With abortions, routine investigations generally include examination of foetal fluid for N. caninum, using an IFAT (immunofluorescent antibody test). If positive, fixed foetal brain (or cardiac tissue if brain is unavailable) is examined for lesions. The combination of foetal serology and lesions is used to make a diagnosis. As N. caninum transmission is vertical, many healthy foetuses will be antibody positive so the presence of other abortifacient agents (such as BVDV) should be considered as the primary agent of abortion if found (Innes et al., 2000). Clinical diagnosis may involve consideration of the whole herd’s health status, with confirmation of N. caninum as the cause of abortion only in the absence of other agents.
Neospora caninum and effects on production
It has been estimated that 12.5% of abortions in dairy cattle in England and Wales, i.e., about 6000 per year, may be attributable to N. caninum although some abortions due to N. caninum may be undetected (Boger and Hattel, 2003; Davison et al., 1999). Most abortions occur either sporadically on farms with annual abortion rates of less than 3% or as more frequent abortions on farms with annual abortion rates of 5 to 10 per cent (Haddad et al., 2005; Taylor, 2000). Occasionally, abortion storms may occur, where rates may reach 60% of cows at risk and may be associated with introduction of infection (horizontal transmission) or by recrudescence of infection in endemically infected herds (Haddad et al., 2005). It is possible for cattle that have previously aborted due to N. caninum infection to have a repeat abortion, with seropositive cows two to 24 times more likely to abort than seronegative cows (Haddad et al., 2005; Hall et al., 2005; Hernandez et al., 2002; López-Gatius et al., 2004; Pfeiffer et al., 2002; Weston et al., 2005). The factors that enhance the likelihood of a seropositive cow aborting remaining largely unknown, although stress and concurrent disease have been suggested (Haddad et al., 2005).
Infection is thought to reduce milk production in adult dairy cows through its effects on fertility as cows which have aborted often produce a lower milk yield and are more likely to suffer retained foetal membranes (Hobson et al., 2002). Seropositive cows that do not abort may not have lower yields though. A high abortion incidence in the herd may lead to an increase in involuntary culling. N. caninum infections in young calves have been reported to cause neuromuscular disease and may affect growth rate of finishing animals (Barling et al., 2001).
The overall economic impact of N. caninum infection has been estimated as US$35 million per year to the Californian dairy industry. N. caninum abortions are incurring losses in excess of AUS$100 million per year in Australia, and €9.7 million per year in Switzerland (Reichel et al., 2014). Direct losses are also suffered due to, infertility, returns to service and loss of milk yield and indirect costs through replacements.
Control and Prevention of Neospora caninum
Neospora caninum and uninfected herds
With uninfected herds, strict biosecurity should be maintained to prevent disease entry. This should form part of the biosecurity component of the Herd Health Plan. Bought in female breeding animals should come from herds known to be free or tested before they calve for the first time. Additionally, risk factors for horizontal transmission should be minimised, so that access to feed and water areas by dogs should be prohibited. A monitoring program should also be undertaken to examine all abortions (including maternal serology) to monitor herd disease status.
Neospora caninum and infected herds

Selecting seronegative heifers as replacements and culling serpositive ones has been shown to reduce disease transmission in Australian herds (Hall et al., 2005)
Neospora caninum-infected cattle are assumed to be infected for life. With infected herds, the aims are to prevent abortion and to reduce the risk of both horizontal and vertical transmission, such that prevalence is reduced in the long-term (Haddad et al., 2005). Ideally, a control policy would be based on knowing the herd seroprevalence of infection and this may be considered if the abortion rate is high. It has been suggested that control of N. caninum infection can be achieved in infected herds by blood testing all dams pre-calving or calves soon after birth and identifying the dams of the seropositive calves as infected animals. These cows can either be culled or bred for beef production only (Penny, 1999). Mathematical modelling has shown that culling of infected cows annually and not breeding replacements from the seropositive offspring will reduce the infection in a herd rapidly if the horizontal transmission by dogs can be limited to a minimum (French et al., 1999). However, N. caninum infected cattle should not be culled automatically as there may be other factors that influence a culling decision such as milk production, genetic merit, udder health (Peregrine et al., 2004) or lameness and not all pregnancies in a seropositive cow will end in abortion despite the increased risk. Farmers should be aware that a seropositive cow while no direct threat to her herdmates acts as a potential reservoir of infection after calving to dogs which can then infect other breeding animals (Haddad et al., 2005; Weston et al., 2005). Based on a modelling approach in beef herds, only breeding from seronegative cows or heifers appears to be a reasonable control strategy (Larson et al., 2004).

Contaminated water with oocysts from dog or other canid faeces may be the source of infection in cattle.
Embryo transfer has been shown to be effective in breaking the lifecycle for cattle with high genetic value (Campero et al., 2003).
Methods of preventing horizontal transmission:
- Prohibit access of dogs to feed storage areas and feeding troughs
- Dispose of placentae and foetuses carefully and promptly after all calvings and abortions
- Do not feed raw bovine meat to farm dogs
- Minimise access by dogs onto pastures (public footpaths) – where unavoidable put up notices for dog owners to pick up dog faeces
- Don’t allow hunts on pastures grazed by breeding stock
A vaccine was available to help control neosporosis called Neoguard™ but it has been withdrawn from global sales due it its questionable efficacy.
Treating Neospora caninum in Cattle
There is no effective treatment for this condition, although aborting cows should be given symptomatic supportive treatment and isolated until a diagnosis is made or veterinary advice given if no diagnosis is reached.
However, dogs can be treated for N. caninum if diagnosed early but you need to consult your vet.
Neospora caninum and Welfare
There are no specific welfare considerations associated with the condition, although efforts should be made to minimise the incidence of abortion in a herd as aborting animals often have subsequent disease problems of retained foetal membranes and endometritis.
Good Practice Based on Current Knowledge
- Investigate all cases of abortion in order to identify causes (See Abortion) – In the UK it is statutory to report all abortions to the Animal and Plant Health Agency (APHA)
- If N. caninum is detected on the farm as a result of an abortion investigation, monitor all calves that are born for seropositivity and try to keep seronegative calves as replacements
- Consider culling infected animals. Do not rear seropositive offspring and/or offspring of infected dams for breeding
- Prevent cows’ access to dogs
- Prevent dogs from soiling pastures, animal feed and water supplies
- Prevent dogs’ access to potentially contaminated material, i.e. afterbirth, aborted materials
- Follow good biosecurity practices to avoid N. caninum coming onto your farm in imported stock
- Keep on top of concomitant infections such as IBR and BVD
- Consider formal eradication or monitoring programme such as the Cattle Health Certification Standards Scheme in the UK which recently included Neospora


 American English
American English



Comments are closed.